Table 1.
Cyclopropanation methods for the preparation of esters 3a–k
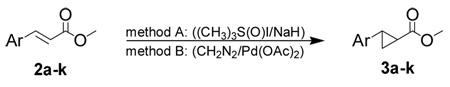 | ||||
|---|---|---|---|---|
| entry | compound | Ar | % yielda, (method A)4 | % yielda, (method B)5 |
| 1 | 3a | 4-CF3C6H4 | 27 | 96 |
| 2 | 3b | 4-BrC6H4 | 50 | 94 |
| 3 | 3c | 4-CH3OC6H4 | 50 | 98 |
| 4 | 3d | 4-NO2C6H4 | 5 | 65 |
| 5 | 3e | 2-thienyl | 40 | 97 |
| 6 | 3f | 3-thienyl | 59 | 99 |
| 7 | 3g | 2-furyl | 27 | 94 |
| 8 | 3h | 3-furyl | 21 | 99 |
| 9b | 3i | 3-pyridyl | 34 | - |
| 10b | 3j | 1-napthyl | 65 | - |
| 11c | 3k | N(Ts)-5-indoyl | - | 97 |
Yields are for isolated product using 1.4 – 1.8 mmol of alkene substrate.
Cyclopropanation by Method B gave inseparable and/or complex mixtures.
Cyclopropanation by Method A not attempted on this substrate.
