Abstract
Background
Atherosclerosis is an inflammatory process that develops in individuals with known risk factors that include hypertension and hyperlipidaemia, influenced by diet. However, the interplay between diet, inflammatory mechanisms and vascular risk factors requires further research. We hypothesised that interleukin-1 (IL-1) signaling in the vessel wall would raise arterial blood pressure and promote atheroma.
Methodology/Principal Findings
Apoe−/− and Apoe−/−/IL-1R1−/− mice were fed high fat diets for 8 weeks, and their blood pressure and atherosclerosis development measured. Apoe−/−/IL-R1−/− mice had a reduced blood pressure and significantly less atheroma than Apoe−/− mice. Selective loss of IL-1 signaling in the vessel wall by bone marrow transplantation also reduced plaque burden (p<0.05). This was associated with an IL-1 mediated loss of endothelium-dependent relaxation and an increase in vessel wall Nox 4. Inhibition of IL-1 restored endothelium-dependent vasodilatation and reduced levels of arterial oxidative stress.
Conclusions/Significance
The IL-1 cytokine system links atherogenic environmental stimuli with arterial inflammation, oxidative stress, increased blood pressure and atherosclerosis. This is the first demonstration that inhibition of a single cytokine can block the rise in blood pressure in response to an environmental stimulus. IL-1 inhibition may have profound beneficial effects on atherogenesis in man.
Introduction
Drugs to decrease plasma lipid levels and blood pressure are the basis of the medical treatment strategy for cardiovascular disease. Dietary modification of macronutrient and salt intakes has also been studied with some large effects upon systolic blood pressure in particular [1]–[3]. However, the exact interplay between the well-described plaque-based inflammatory processes of atherogenesis [2], [4] and the conventional risk factors of lipids and blood pressure remains poorly understood. We hypothesized that the pro-inflammatory cytokine interleukin-1 (IL-1), known to have an association with atherosclerosis, is the link between vascular responses and high fat feeding.
In the arterial wall, IL-1 is secreted primarily by monocytes and macrophages but also by endothelial and smooth muscle cells [5], [6]. IL-1 is an apical cytokine initiating inflammatory signals from bacterial products, chemical injury, complement activation and clotting factors. There are two agonistic cytokines, IL-1α and β which both signal via the type-I IL-1 receptor (IL-1R1). The system is tightly regulated by a natural antagonist, interleukin-1 receptor antagonist (IL-1ra) which also binds to IL-1R1 but does not produce a signal, and a non-signaling cell surface receptor, IL-1 receptor type-II, which acts as a decoy at the cell membrane or when shed as a soluble circulating decoy receptor [7], [8].
IL-1 is a powerful inflammatory stimulus to endothelial and vascular smooth muscle cells with effects that are plausibly linked with known mechanisms of atherogenesis [9]. Levels of IL-1 are increased in coronary arteries affected by atherosclerosis [10], [11], and inhibition of IL-1 in animal models is associated with reduced amounts of atheroma [12]–[16] as well as neointima following angioplasty [17]. Excess IL-1 leads to vascular cell oxidative stress, which is linked with elevation of arterial blood pressure mediated by adverse effects of oxidative stress upon bioavailable nitric oxide.
We report here the cardiovascular effects of abolishing IL-1 signaling either by genetic deletion of IL-1R1 or by administration of IL-1ra in proatherogenic Apoe−/− mice fed atherogenic diets. We also show a link between fat feeding and blood pressure that is mediated via IL-1 through modulation of the NADPH-oxidase subunit 4 (Nox 4).
Materials and Methods
More detailed methods can be found in the online supplemental material (Text S1).
Animals
Apoe−/−/IL-R1−/− mice were generated at JAX labs by cross breeding of Apoe−/− (JAX 2052) with IL-R1−/− (JAX 3245) mice. All mice were on a C57BL/6 background. Male mice, 8 weeks of age, (12 per group) were fed normal chow (4.3% fat, 0.02% cholesterol), Western diet (21% fat, 0.15% cholesterol, 0.03% cholate, 0.296% sodium) or Western High Cholate (WHC or Paigen) diet (18.5% fat, 0.9% cholesterol, 0.5% cholate, 0.259% sodium) for 8 weeks. No differences in body weights between the strains were observed either with or without fat feeding. Diets were supplied by Special Diet Services, UK. All animal experiments were approved by the University of Sheffield Project Review Committee and conformed to UK Home Office ethical guidelines.
Quantification of Atherosclerotic Lesions
Histologically stained paraffin wax embedded sections were analysed as previously described [18].
Blood Pressure Analysis
Systolic and diastolic blood pressures of mice were measured using a Visitech tail-cuff system (Visitech Systems, NJ, USA) and mean blood pressure calculated (supplementary text S1).
Arteriolar Myogenic Reactivity and Responsiveness to Nitric Oxide
A pressure myograph system (Living Systems Instrumentation, USA) was used to determine arteriolar myogenic reactivity and responsiveness to nitric oxide, as previously described [19], [20].
Bone Marrow transplantation
Irradiation and bone marrow transplantation of mice was performed as previously described [18] (text S1).
Detection of Reactive Oxygen Species (ROS), NOS and Nitric Oxide
Superoxide production in mouse aortic tissue sections was detected in-situ [21]. Levels of ROS in aortic tissue were determined by chemiluminescent assay [22]. Nitric oxide synthase (NOS) enzymatic activity, and indirectly nitric oxide (NO) synthesis, was measured by the conversion of 14C L-arginine to 14C L-citrulline [23]. These methods are given in detail in the supplementary data. NO bioactivity in mice fed WHC was determined using an enzyme immunoassay for cyclic guanosine monophosphate (cGMP) (Cayman Chemical, UK).
Quantitative Real time polymerase chain reaction
Quantification of Nox 2 and Nox 4 was performed by amplification of artery cDNA using a MX3000P instrument (Stratagene) with SYBR green dye, and normalization to 18S rRNA. For cultured cells, normalization was to β-actin. Optimized amplification conditions were 150 mmol/L primers, 2.5 mmol/L MgCl2, annealing at 60°C and extension at 72°C. Primer sequences were as described in [24].
Statistical Analysis
Data groups were analyzed by t-test or one-way ANOVA followed by a Bonferroni multiple comparison post-test, as appropriate. Blood pressure measurements were analyzed by global non-linear regression, followed by an F-test. Vessel diameters were analyzed by two-way repeated-measures ANOVA. pD2, Emax, L-NAME effects and wall: lumen ratios were compared by t-tests. Data are presented as mean±SEM. p<0.05 was regarded as a significant difference.
Results
Further results can be found in the online supplementary text (Results S1).
Effects of genetic deletion of IL-1R1 and diet type on atherosclerosis lesion development
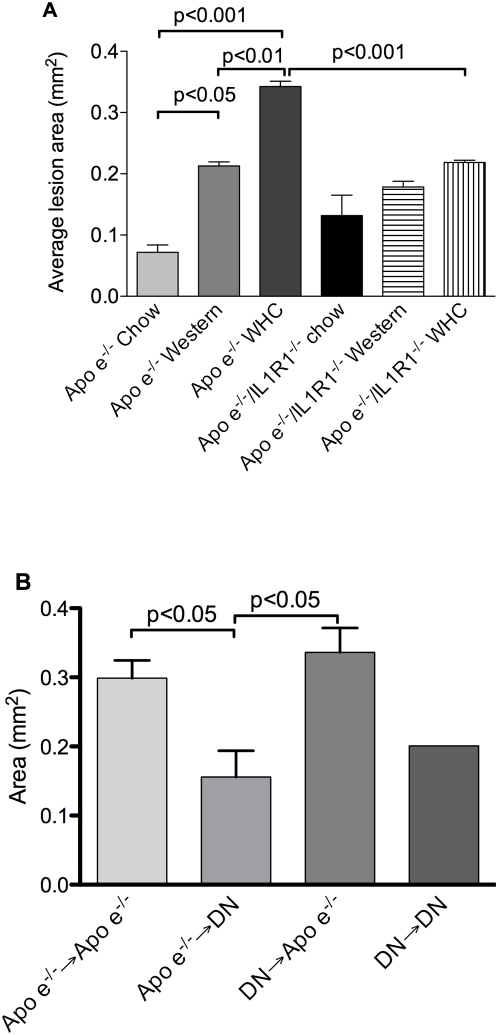
As expected, Apoe−/− mice fed high fat diets displayed significant increases in atherosclerotic lesion area compared to mice fed chow, with the Western diet with high cholate (WHC) causing the a greatest increase (Figure 1a). In contrast, lesions developed by Apoe−/−/IL-R1−/− mice fed these high fat diets were not significantly different to Apoe−/−/IL-R1−/− mice fed on chow. Analysis of each different diet type separately revealed no significant difference in lesion area of Apoe−/− and Apoe−/−/IL-R1−/− mice fed chow (p = 0.3), or Western diet (p = 0.4). However, when fed the WHC diet, Apoe−/−/IL-R1−/− mice developed a 36% smaller atherosclerotic lesion in the aortic sinus compared to Apoe−/− mice (p<0.001) (Figure 1a). Representative images of the microscopic appearance of the lesions are shown in Figure S1. Characterisation of the lesions revealed no overt differences with respect to strain or diet (Results S1, table S1, Table S2, figure S2). Body weight did not differ between mice of any group (data not shown).
Figure 1. Atherosclerosis formation in mice following feeding of a high fat diet.
Atherosclerotic lesion area in the aortic sinus of Apoe−/− and Apoe−/−/IL-R1−/− mice (a) and chimeric mice (b) fed a high fat and chow diets. Apoe→DN denotes Apoe−/−/IL-1R1−/− (DN) mice receiving Apoe−/− bone marrow (n = 3); DN→Apoe denotes Apoe−/− mice receiving Apoe−/−/IL-1R1−/− marrow (n = 9).
Atherosclerotic lesion formation in IL-1R1 chimeric mice fed a WHC diet
Apoe−/− and Apoe−/−/IL-R1−/− chimeric mice were used to determine the relative contribution of IL-1 signaling in cells derived from the bone marrow and non-bone marrow (tissue cells) upon the development of atherosclerosis following fat feeding. Irradiated Apoe−/− mice receiving Apoe−/− bone marrow had large, and Apoe−/−/IL-R1−/− mice receiving Apoe−/−/IL-R1−/− marrow relatively little, atherosclerosis in their aortic sinus following 8 weeks on a WHC diet, as expected and analogous to Apoe−/− and Apoe−/−/IL-R1−/− non-chimeric animals.
Interestingly, Apoe−/− animals receiving Apoe−/−/IL-R1−/− bone marrow (i.e. chimera is capable of IL-1 signaling in vessel wall/tissue cells) did not develop a lesion that was significantly different from Apoe−/ mice receiving Apoe−/− marrow (Figure 1b). In contrast, Apoe−/−/IL-R1−/− mice receiving Apoe−/− bone marrow (i.e. chimera is not able to signal via IL-1 in vessel wall/tissue cells) developed a 1.9-fold smaller lesion than the Apoe−/− to Apoe−/− controls (p<0.05). The lesion size between Apoe−/−/IL-R1−/− into Apoe−/− and Apoe−/− into Apoe−/−/IL-R1−/− mice also differed significantly (p<0.05) (Figure 1b).
Pathological analysis, by chromosome painting, of these animals showed all had engrafted donor marrow (figure S3).
Blood pressure responses to fat feeding
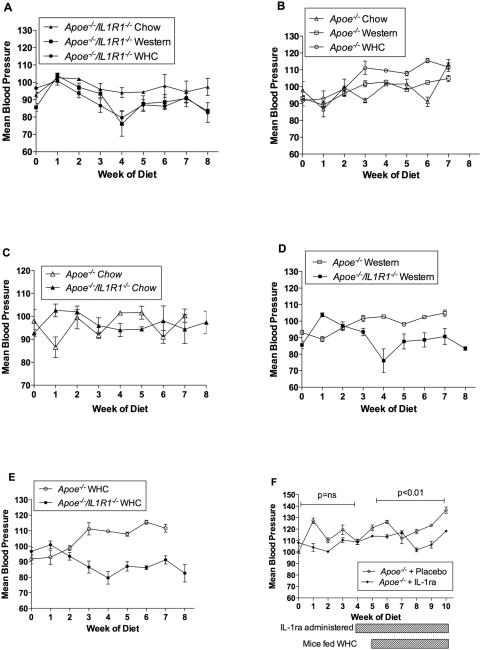
There was no difference in mean blood pressure in Apoe−/−/IL-R1−/− mice fed the Western, WHC or chow diet (figure 2a). However, Apoe−/− mice fed high fat diets had increased blood pressure compared with chow fed animals, with WHC-fed mice having a greater elevation than Western fed animals (p<0.05 WHC vs Western or chow)(figure 2b).
Figure 2. Blood pressure of mice following feeding of a high fat diet.
Mean blood pressure in Apoe−/−/IL-1R1−/− (a) and Apoe−/ (b) mice fed all 3 diets, and Apoe−/−/IL-1R1−/− compared to Apoe−/− mice fed chow (c) Western (d) and WHC (e). (f) Mean blood pressure in Apoe−/− mice administered with hrIL-1ra or placebo, fed WHC diet. n = 4 for all groups.
When comparing mouse strains, no difference was seen in mean blood pressure between Apoe−/−/IL-R1−/− or Apoe−/− fed a normal chow diet over an 8-week period (p = ns)(figure 2c). However, on feeding high fat diets, Apoe−/−/IL-R1−/− mice fed either the Western or WHC diet had a significantly lower blood pressure (a level comparable to Apoe−/− mice on chow diet) than the Apoe−/− mice fed the equivalent diet (p<0.001; p<0.0001 respectively, figure 2d, 2e).
Apoe−/− mice on WHC diets infused with IL-1ra using an osmotic pump also had a significantly lower blood pressure than Apoe−/− mice given placebo (Figure 2f). Elevations of IL-1ra in these animals was confirmed by ELISA, and found to be 1435.1+/−118.7 pg/ml compared to 0.215+/−0.1452 pg/ml in placebo mice.
Effect of IL-1R1 deletion on vascular reactivity and compliance in mice fed WHC diet
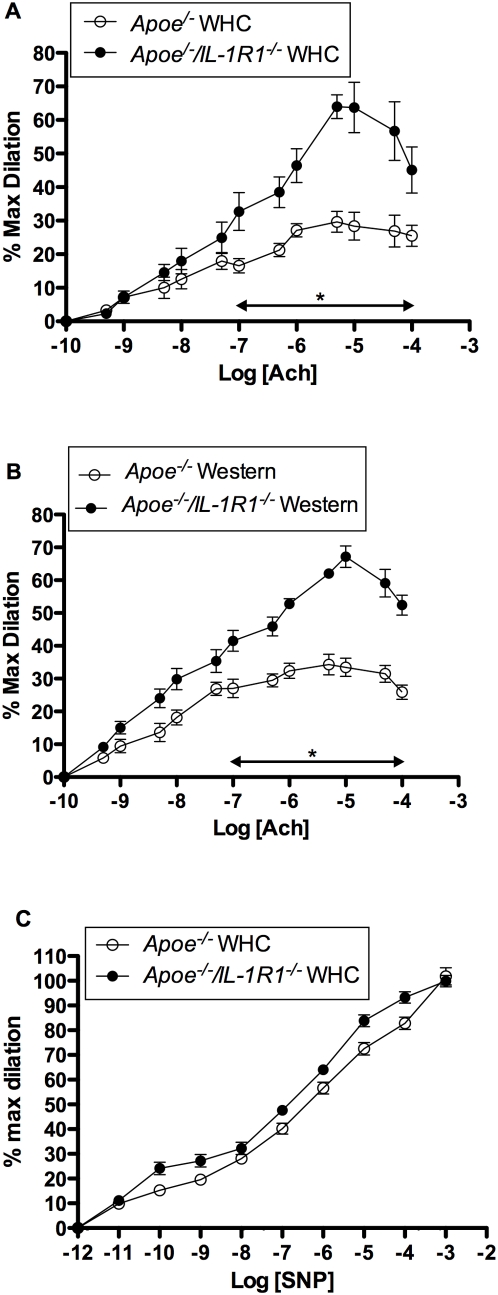
Mesenteric arteries from Apoe−/− and Apoe−/−/IL-R1−/− mice fed Western and WHC diets did not differ in their sensitivity to phenylephrine (PE) (table S3). However, preconstricted arteries from Apoe−/−/IL-R1−/− mice fed these diets displayed significantly greater vasodilator sensitivity to acetyl choline (ACh) (p<0.05; Figures 3a and b) compared to Apoe−/− animals. The vasodilatory response to ACh was also significantly different between chow, Western and WHC diets in Apoe−/− mice (p<0.05). In Apoe−/−/IL-R1−/− mice fed WHC diet there was greater constriction (19.1+/−1.3%) compared to Apoe−/− (8.6+/−2.7%) following incubation with N(G)-nitro-L-arginine methyl ester (L-NAME) (p = 0.003), without a significant difference between pD2 (negative logarithm of EC50: concentration required for half maximum response) (table S3).
Figure 3. Vascular reactivity of arterioles from Apoe−/− and Apoe−/−/IL-R1−/− mice.
(a–b) % maximum dilation of pre-constricted mesenteric arterioles (EC80PE) to acetylcholine. (c) Endothelium-independent relaxation responses assessed by superfusion of sodium nitroprusside (SNP) were not different between Apoe−/− and Apoe−/−/IL-R1−/− mice both fed a WHC diet (n = 6 per group).
Both Apoe−/− and Apoe−/−/IL-R1−/− mice demonstrated similar internal luminal diameters of small mesenteric arteries increased in response to increasing intraluminal pressure (active response), with Apoe−/− mice fed either diet showing a trend towards a reduced myogenic response (figure S4a, S4b). However, only Apoe−/−/IL-R1−/− mice fed WHC diet demonstrated a greater active pressure-diameter response compared to chow fed Apoe−/− mice (figure S4a).
There was no difference in the wall-to-lumen ratio (vascular smooth muscle thickness/external diameter at 80 mmHg; h/R) [19] between the two groups (0.115+/−0.007 Apoe−/−/IL-R1−/− vs 0.111+/−0.011 Apoe−/−, n = 6). Endothelium-independent relaxation responses, assessed by superfusion of sodium nitroprusside (SNP), also did not differ (figure 3c).
Effect of IL-1R1 deletion on NO activity, ROS, NOS and NOX expression in mice fed WHC diet
The induction of Nox4 mRNA and generation of ROS in response to IL-1 stimulation was confirmed in arteriolar endothelial cells (Figure S5). ROS were not generated in response to IL-1 in endothelial cells isolated from Apoe−/−/IL-R1−/− mice (figure S5). In contrast, Nox 4 mRNA was reduced in IL-1 stimulated vascular smooth muscle cells (VSMC) (figure S6) as previously shown [25].
The vessel wall mechanisms that could contribute to the observed vascular reactivity effects were investigated by examining the expression of NO, ROS, NOS and Nox isoforms in WHC diet-fed animals, as this diet gave the largest difference in blood pressure and atheroma between animals with and without the ability to signal via IL-1R1.
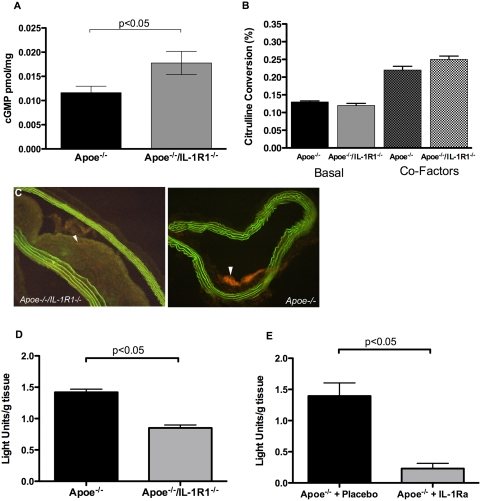
NO bioactivity was increased in Apoe−/−/IL-R1−/− mice fed a WHC diet compared with WHC fed Apoe−/− mice (n = 9–11, p<0.05) (Figure 4a). Activity of eNOS in the presence or absence of eNOS co-factors did not differ between Apoe−/−/IL-R1−/− mice fed a WHC diet compared with Apoe−/− mice (p = ns) (Figure 4b). Qualitative in situ detection of ROS showed production in atherosclerotic plaques was reduced in Apoe−/−/IL-R1−/− compared to Apoe−/− mice fed WHC diet (Figure 4c). Quantitative measurement of aortic ROS production showed ROS to be significantly reduced in Apoe−/−/IL-R1−/− mice on a WHC diet (n = 9, p<0.05)(Figure 4d). ROS production was also suppressed in Apoe−/− mice on a WHC diet by a 4-week infusion of IL-1ra (n = 3, p<0.05)(Figure 4e). Generation of ROS in response to feeding a high fat diet was also confirmed (figure S7). Apoe−/− mice fed WHC had significantly more ROS than those fed chow alone, with Western diet giving intermediate levels of ROS. ROS levels were also significantly higher in Apoe−/−/IL-R1−/− mice fed WHC compared to chow (0.85+/−0.14 compared to 0.51+/−0.30 LU/g tissue, n = 9, p<0/01).
Figure 4. NO activity, ROS and NOS expression in mice fed WHC diet.
(a) cGMP in lung homogenates (n = 14), (b) eNOS enzymatic activity (n = 6) and (c) in-situ ROS (white arrowhead) in Apoe−/−/IL-R1−/− and Apoe−/− mice fed WHC. (Note: images chosen illustrate ROS levels not atherosclerotic lesion size). Chemiluminescent quantification of ROS in mice fed WHC (d) and Apoe−/− mice fed WHC diet (n = 9) and administered hrIL-1ra (n = 4) (e).
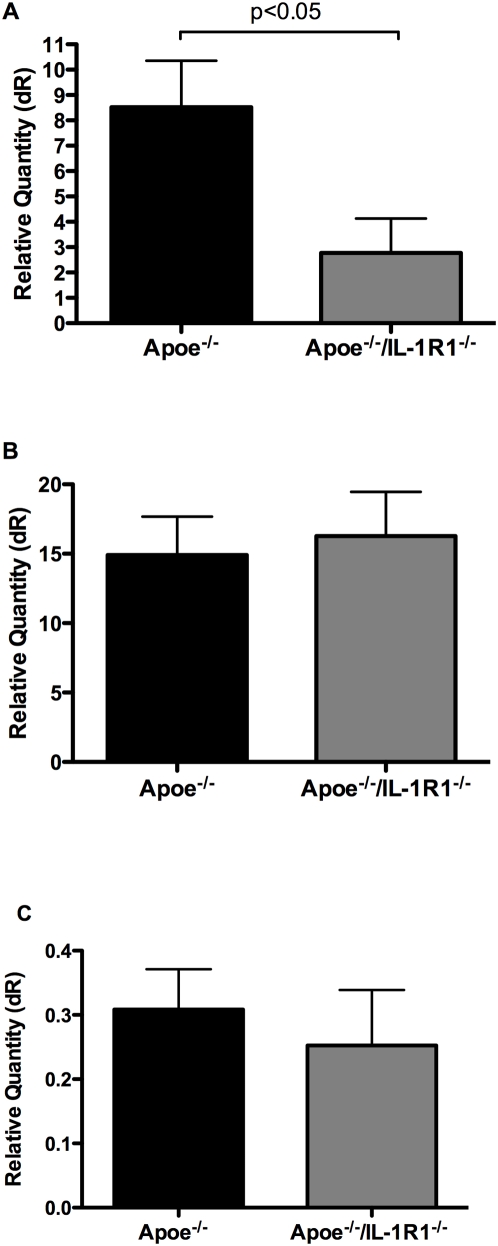
Nox 4 expression in the whole arterial wall was significantly reduced in Apoe−/−/IL-R1−/− mice fed the WHC diet compared to Apoe−/− mice (Figure 5a). Expression of Nox 1 and 2 however, did not differ between these two mouse strains on this diet (figure 5b, 5c).
Figure 5. Nox expression following feeding of WHC diet.
Expression of Nox is selectively reduced in Apoe−/−/IL-R1−/− mice fed WHC. (a) qRT-PCR shows reduced (p<0.05) levels of Nox 4 in the vascular wall of Apoe−/−/IL-R1−/− mice fed WHC diet. Levels of Nox 2 (b) and Nox 1 (c) do not differ. n = 12 per group.
Discussion
The Apoe−/− mouse fed a high fat diet is an established model of atherogenesis. On a Western or a Western high cholate diet (WHC, sometimes called the Paigen diet), Apoe−/− mice develop aortic atheroma [26] and blood pressure is known to rise within 1 to 3 months of feeding. We show here that IL-1 inhibition reduces atheroma formation and prevents the associated rise in blood pressure in Apoe−/− mice fed high fat diets.
IL-1 inhibition was achieved by generating mice deficient for both Apoe and IL-1 receptor 1 (IL-1R1) genes. As expected, aortic atheroma was significantly reduced in Apoe−/−/IL-R1−/− mice fed WHC compared with Apoe−/− controls. This is in agreement with previous studies that showed decreased atherosclerosis in IL-1β−/− and IL-1α−/− mice fed a high cholate diet [27] and with data from a heterozygote Apoe−/− model [16]. In addition, both high fat diets were associated with increased markers of inflammation that were attenuated in Apoe−/−/IL-1R1−/− mice (Results S1). Interestingly, IL-1β and IL-1ra plasma levels in mice were not changed in response to fat-feeding, This was not unexpected: mature plasma IL-1β is a difficult parameter to interpret as a non-secreted protein and IL-1ra behaves as an acute phase reactant with a large variation in levels [28]. Any true changes in inflammatory markers are more likely to occur locally within the vessel wall itself.
Apoe−/− mice fed a high fat diet display a rise in blood pressure that is not seen in Apoe−/−/IL-R1−/− mice fed equivalent diets. The specificity of this was confirmed by abolition of the fat-feeding induced blood pressure rise in Apoe−/− mice given IL-1ra. Stroke-prone spontaneously hypertensive rats (SHRSP) showed no difference in blood pressure if given IL-1ra (data not shown), suggesting that IL-1ra does not act as a non-specific vasodilator.
To study the mechanism of the attenuation of the blood pressure rise in the Apoe−/−/IL-R1−/− mice we investigated active arteriolar pressure-diameter responses and basal and agonist-induced NO production in Apoe−/− and Apoe−/−/IL-R1−/− fat-fed mice. The greater vessel diameter in response to increasing pressure in vessels from Apoe−/−/ILR1−/−, together with no observed difference in sensitivity to PE between groups or in vascular smooth muscle constriction for the given degree of muscle shortening (wall: lumen ratio), as well as the preserved response to SNP, all suggest a specific endothelial response.
The strikingly preserved agonist-induced NO dependent vasodilatation of resistance vessels from Apoe−/−/IL-1R1−/− mice compared to Apoe−/− in both diet groups as well as the greater contraction in response to L-NAME in the fat-fed Apoe−/−/IL-1R1−/− mice indicate a fundamental increase in bioavailable vessel wall nitric oxide as a result of selective loss of IL-1 signaling. This is emphasised by the observed increase in basal cGMP production seen in these mice. The activity of eNOS, in the presence or absence of eNOS co-factors, did not differ between the two mouse strains suggesting that the difference in bioavailable NO attributable to selective IL-1 inhibition is mediated by increased consumption of NO via increased arterial oxidative stress.
In support of these data, there was consistent reduction of arterial ROS levels in mice unable to signal via IL-1. Investigation of Nox isoforms, known to regulate NADPH activity [29], [30], indicated a Nox-4 selective rise in response to fat feeding in the Apoe −/− mouse attenuated by abolition of IL-1 signaling. Taken together, these results suggest a central role for IL-1 in modulating the blood pressure response to high lipids and that this is via a selective reduction in bioavailable nitric oxide.
The chimeric mouse experiments were conducted to examine the compartment in which IL-1 signaling was important. Only in chimeras where tissue cells were unable to signal via IL-1 was a there a decrease in atherosclerotic lesion size. These data complement a recent study by Kamari et al. who showed reduced atherosclerosis in wild type, WHC-fed mice reconstituted with either IL-1β−/− or IL-1α−/− bone marrow [27].
We also demonstrate that vessel wall ROS production and Nox 4 levels are altered between the non-chimeric Apoe−/− and Apoe−/−/IL-1R1−/− strains on high fat diets. The myography experiments also suggest a selective endothelial loss of function. Thus, the data presented here add to the hypothesis that IL-1 signaling within the blood vessel wall cells is the mechanism involved in the blood pressure and atherogenic responses to fat feeding in Apoe−/− mice.
Our studies examined the effects of two different pro-atherogenic diets, administered for 8 weeks. Of these diets, the WHC diet is known to be the most aggressive, giving a chronic inflammatory response compared to the acute response of a standard Western (high cholesterol/low cholate) diet [31]. Besides being a normal component of bile in vertebrates, cholate (E1000) is an emulsifier and foam stabilizing agent used in food manufacturing which leads to very high circulating cholesterol concentrations in plasma. Given the difference in plasma cholesterol load, it is interesting that the poor vascular reactivities of vessels from mice fed both diets were similar with a clear gain of function seen in mice lacking the ability to signal via IL-1R1, irrespective of the cholate content. Atheroma development and blood pressure elevation were graded in magnitude through chow to the Western and WHC diets, with the WHC having the greatest effect. In keeping with this, the greatest level of attenuation of biological events by IL-1 inhibition was seen in the WHC diet fed animals. These all suggest a specificity of IL-1 in the mediation of fat fed vascular events.
The WHC diet can cause intense metabolic disruption, hepatic dysfunction and inflammatory activation. We did not see these effects. Additionally, we saw similar index data (e.g. blood pressure and atheroma formation) for mice fed on the Western and the WHC diet, though the magnitude of the blood pressure effects was reduced with the Western diet. We feel, therefore, that where we have used the WHC diet for further detailed experimentation that this is justified and that the results derived are the result of elevation in cholesterol: the Western diet giving intermediate cholesterol levels and intermediate phenotype, and the WHC diet giving rise to high cholesterol and an advanced phenotype.
There is considerable interest in harnessing knowledge of basic inflammatory mechanisms of atherogenesis for therapeutic benefit. We show that dietary manipulation in a murine model of atherosclerosis is associated with a rise in blood pressure, an effect attenuated by selective inhibition of a single cytokine. High fat diets may be particularly linked with elevation in blood pressure [3], [32]. It is clear that in humans, dietary alterations away from a standard Western diet to those lower in fat, but which are isocaloric and balanced for sodium content, or which are supplemented with omega-3 fatty acids, also lower blood pressure [1], [33]–[35]. Omega-3 fatty acids have also been shown to reduce cyclooxygenase-2 and NADPH oxidase activity [36], both of which are induced by IL-1. This suggests that the link between diet and blood pressure may be through the IL-1 cytokine system, and may indicate a role for IL-1 inhibition in the control of hypertension in man.
In our experiments, there was a concomitant reduction in blood pressure and atheroma development. In fat-fed Apoe−/− mice, development of atheroma is not driven directly by the rise in blood pressure [37]. It seems likely therefore, that the IL-1 mediated increase in ROS and decreased NO production are the central mechanism of atheroma development as both of these have individually been implicated in atherogenesis. The absence of difference in lipid levels between the different mouse strains on the same diets (results S1) confirms the likely effect of IL-1 inhibition on atherogenesis being a ROS/NO-based mechanism.
These data raise the possibility of an entirely novel strategy for prevention of atherosclerosis. An anti-IL-1 based approach would be expected to have a beneficial effect upon atherogenic mechanisms at multiple levels. Indeed, very recent data have indicated a beneficial effect of IL-1ra upon patients with type II diabetes [38], ventricular function [39] and myocardial infarction in mice [40] and a similar approach might allow mitigation of the atherogenic potential of Western diets. We anticipate that targeted pharmacological intervention to inhibit IL-1 in man will be beneficial in preventing atheroma development and the clinical consequences that arise from this important disease of developed nations.
Supporting Information
Expanded methods.
(0.04 MB DOC)
Additional data
(0.04 MB DOC)
Microscopic appearances around the aortic sinus of ApoE−/− mice fed chow, Western, and WHC compared with ApoE−/−/IL-1R1−/− mice. Original magnification×2.
(1.18 MB TIF)
Modulation of IL-1 signaling decreases acute phase reactant serum amyloid A (SAA) levels. SAA was elevated in Apoe−/− mice on both high fat diets, an increase that was significantly reduced in the Apoe−/−/IL-R1−/− mice on equivalent diets. (n = 9–20)
(4.80 MB TIF)
Chromosome painting of bone-marrow transplanted mice. No Y-chromosomes were seen in male mice transplanted with female bone marrow, confirming engraftment was successful. Male recipients of male bone marrow all have Y-chromosomes, as expected, as a positive control for this method.
(2.45 MB TIF)
Vascular reactivity of arterioles from Apoe−/− and Apoe−/−/IL-R1−/− mice. Intraluminal arteriolar diameter in response to increasing pressure (0–120 mmHg). *P<0.05 Apoe−/−/IL-R1−/− (n = 6) and **P<0.05 Apoe−/− WHC (n = 6) versus Apoe−/− chow (n = 4).
(0.61 MB TIF)
ROS and Nox 4 expression in endothelial cells in culture. Increased ROS are seen following IL-1β stimulation of endothelial cells isolated from Apoe−/− mice (n = 6) (a) and human coronary endothelial cells (hCAEC) (n = 6) (b). However, no increase in ROS are seen in ECs isolated from Apoe−/−/IL-1R1−/− mice (n = 6) (a). Nox 4 mRNA expression is increased in hCAEC stimulated with IL-1β (n = 5) (c).
(9.90 MB TIF)
ROS and Nox 4 expression in vascular smooth muscle cells in culture. No significant difference in ROS are seen following IL-1β stimulation of VSMCs (n = 6) (a). Nox 4 mRNA expression is decreased in VSMC stimulated with IL-1β (n = 1) (b).
(0.30 MB TIF)
ROS generation in Apoe−/− mice following feeding of a high fat diet. Mice fed WHC had significantly more ROS than those fed chow alone, with Western diet giving intermediate levels of ROS. (n = 9).
(2.79 MB TIF)
Lipid, glucose and ALT levels in ApoE−/−/IL-1R1−/− and ApoE−/− mice fed chow, Western High Cholate (WHC), and Western diets. Data represents mean+/−SEM.
(0.05 MB DOC)
Plasma levels of IL-1ra, IL-1β, IL-1α and IL-6 in ApoE−/−/IL-1R1−/− and ApoE−/− mice fed chow, Western high cholate (WHC), and Western diets, determined by ELISA. Data represents mean+/−SEM.
(0.04 MB DOC)
Potency and efficacy of vasoconstrictor response to phenylephrine in pressurised mesenteric arterioles from Apoe−/−/IL1R1−/− and Apoe−/− mice fed a Western high cholate (WHC), Western, or Apoe−/− mice fed chow diet, assessed by pD2 (negative logarithm of EC50: concentration required for half maximum response) and Emax (percentage maximum constriction). All data are means+SEM. p = ns.
(0.04 MB DOC)
Acknowledgments
The authors thank Nadine Arnold, Dr Andrea King, Dr Victoria Ridger, Dr Karim Jetha (Univ Sheffield), Dr Kim Suvarna (Department of Histopathology, Northern General Hospital, Sheffield, UK) for their contribution towards this manuscript. Amgen Inc provided the IL-1ra used in this study.
Footnotes
Competing Interests: Authors SEF and DCC hold limited stock in ILG.
Funding: This study was supported by British Heart Foundation Programme Grant RG 2001/008. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1.Appel LJ, Sacks FM, Carey VJ, Obarzanek E, Swain JF, et al. Effects of protein, monosaturated fat, and carbohydrate intake on blood pressure and serum lipids: results of the OmniHeart randomized trial. JAMA. 2005;294:2455–2464. doi: 10.1001/jama.294.19.2455. [DOI] [PubMed] [Google Scholar]
- 2.Hansson GK. Inflammation, Atherosclerosis and Coronary artery Disease. N Engl J Med. 2005;352:1685–1695. doi: 10.1056/NEJMra043430. [DOI] [PubMed] [Google Scholar]
- 3.Dickinson HO, Mason JM, Nicolson DJ, Campbell F, Beyer FR, et al. Lifestyle interventions to reduce raised blood pressure: a systematic review of randomized controlled trials. J Hypertens. 2006;24:215–233. doi: 10.1097/01.hjh.0000199800.72563.26. [DOI] [PubMed] [Google Scholar]
- 4.Glass CK, Witztum JL. Atherosclerosis: The Road Ahead. Cell. 2001;104:503–516. doi: 10.1016/s0092-8674(01)00238-0. [DOI] [PubMed] [Google Scholar]
- 5.Bevilacqua MP, Pober JS, Majeau GR, Cotran RS, Gimbrone MA. Interleukin-1 induces biosynthesis and cell surface expression of procoagulant activity in human vascular endothelial cells. J Exp Med. 1984;160:618–623. doi: 10.1084/jem.160.2.618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Libby P, Ordovas JM, Birinyi LK, Auger KR, Dinarello CA. Inducible interleukin-1 gene expression in human vascular smooth muscle cells. J Clin Invest. 1986;78:1432–1438. doi: 10.1172/JCI112732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Colotta F, Dower SK, Sims JE, Mantovani A. The Type II ‘decoy’ receptor ; a novel regulatory pathway for IL-1. Immunol Today. 1994;15:562–526. doi: 10.1016/0167-5699(94)90217-8. [DOI] [PubMed] [Google Scholar]
- 8.Symons JA, Young PR, Duff GW. Soluble type II IL-1 receptor binds and blocks processing of IL-1beta precursor and loses affinity for IL-1ra. Proc Natl Acad Sci USA. 1995;92:1714–1718. doi: 10.1073/pnas.92.5.1714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tamaru M, Tomura K, Sakamoto S, Tezuka K, Tamatani T, et al. Interleukin-1 beta induces tissue- and cell-specific expression of adhesion molecules in vivo. Arterioscler Thromb Vasc Biol. 1998;18:1292–1303. doi: 10.1161/01.atv.18.8.1292. [DOI] [PubMed] [Google Scholar]
- 10.Galea J, Armstrong J, Gadsdon P, Holden H, Francis SE, et al. IL-1beta in the coronary arteries of patients with ischaemic heart disease. Arterioscler Thromb Vasc Biol. 1996;16:1000–1006. doi: 10.1161/01.atv.16.8.1000. [DOI] [PubMed] [Google Scholar]
- 11.Dewberry R, Holden H, Crossman D, Francis S. Interleukin-1 receptor antagonist expression in human endothelial cells and atherosclerosis. Arterioscler Thromb Vasc Biol. 2000;20:2394–2400. doi: 10.1161/01.atv.20.11.2394. [DOI] [PubMed] [Google Scholar]
- 12.Kirii H, Niwa T, Yamada Y, Wada H, Saito K, et al. Lack of interleukin-1β decreases the severity of atherosclerosis in Apo-E deficient mice. Arterioscler Thromb Vasc Biol. 2003;23:656–660. doi: 10.1161/01.ATV.0000064374.15232.C3. [DOI] [PubMed] [Google Scholar]
- 13.Elhage R, Maret A, Pieraggi MT, Thiers JC, Arnal JF, et al. Differential effects of interleukin-1 receptor antagonist and tumor necrosis factor binding protein on fatty-streak formation in apolipoprotein E-deficient mice. Circulation. 1998;97:242–244. doi: 10.1161/01.cir.97.3.242. [DOI] [PubMed] [Google Scholar]
- 14.Isoda K, Sawada S, Ishigami N, Matsuki T, Miyazaki K, et al. Lack of interleukin-1 receptor antagonist modulates plaque composition in apolipoprotein E-deficient mice. Arterioscler Thromb Vasc Biol. 2004;24:1068–1073. doi: 10.1161/01.ATV.0000127025.48140.a3. [DOI] [PubMed] [Google Scholar]
- 15.Devlin CM, Kuriakose G, Hirsch E, Tabas I. Genetic alterations of IL-1 receptor antagonist in mice affect plasma cholesterol level and foam cell lesion size. Proc Natl Acad Sci USA. 2002;99:6280–6285. doi: 10.1073/pnas.092324399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chi H, Messas E, Levine RA, Graves DT, Amar S. IL-1 Receptor signaling mediates atherosclerosis associated with bacterial exposure and/or high fat diet in a murine apolipoprotein E Heterozygote model. Circulation. 2004;110:1678–1685. doi: 10.1161/01.CIR.0000142085.39015.31. [DOI] [PubMed] [Google Scholar]
- 17.Morton AC, Arnold ND, Gunn J, Varcoe R, Francis SE, et al. Interleukin-1 receptor antagonist alters the response to vessel wall injury in a porcine coronary artery model. Cardiovasc Res. 2005;68:493–501. doi: 10.1016/j.cardiores.2005.06.026. [DOI] [PubMed] [Google Scholar]
- 18.Chamberlain J, Evans D, Dower S, Crossman D, Francis S. IL-1β and signaling of IL-1 in vascular wall and circulating cells modulates the extent of neointima formation after vascular injury in mice. Am J Pathol. 2006;168:1396–1403. doi: 10.2353/ajpath.2006.051054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Brookes ZL, Kaufman S. Myogenic responses and compliance of mesenteric and splenic vasculature in the rat. Am J Physiol Regul Integr Comp Physiol. 2003;284:R1604–R1610. doi: 10.1152/ajpregu.00411.2002. [DOI] [PubMed] [Google Scholar]
- 20.Andrew PS, Kaufman S. Guanylyl cyclase mediates ANP-induced vasoconstriction of murine splenic vessels. Am J Physiol Regul Integr Comp Physiol. 2003;284:R1567–R1571. doi: 10.1152/ajpregu.00417.2002. [DOI] [PubMed] [Google Scholar]
- 21.Alp NJ, McAteer MA, Khoo J, Choudhury RP, Channon KM. Increased endothelial tetrahydrobiopterin synthesis by targeted transgenic GTP-cyclohydrolase I overexpression reduces endothelial dysfunction and atherosclerosis in ApoE-knockout mice. Arterioscler Thromb Vasc Biol. 2004;24:445–450. doi: 10.1161/01.ATV.0000115637.48689.77. [DOI] [PubMed] [Google Scholar]
- 22.Laursen JB, Somers M, Kurz S, McCann L, Warnholtz A, et al. Endothelial regulation of vasomotion in ApoE-deficient mice. Implications for interactions between peroxynitrite and tetrahydrobiopterin. Circulation. 2001;103:1282–1288. doi: 10.1161/01.cir.103.9.1282. [DOI] [PubMed] [Google Scholar]
- 23.Alp NJ, Mussa S, Khoo J, Cai S, Guzik T, et al. Tetrahydrobiopterin-dependent preservation of nitric oxide-mediated endothelial function in diabetes by targeted transgenic GTP-cyclohydrolase I overexpression. J Clin Invest. 2003;112:725–735. doi: 10.1172/JCI17786. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Guzik TJ, Sadowski J, Guzik B, Jopek A, Kapelak B, et al. Coronary artery superoxide production and Nox isoform expression in human coronary artery disease. Arterioscler Thromb Vasc Biol. 2006;26:333–339. doi: 10.1161/01.ATV.0000196651.64776.51. [DOI] [PubMed] [Google Scholar]
- 25.Ellmark SH, Dusting GJ, Fui MW, Guzzo-Pernell N, Drummond GR. The contribution of Nox 4 to NADPH oxidase activity in mouse vascular smooth muscle. Cardiovascular Research. 2005;65:495–504. doi: 10.1016/j.cardiores.2004.10.026. [DOI] [PubMed] [Google Scholar]
- 26.Getz GA, Reardon CA. Diet and murine atherosclerosis. Arterioscler Thromb Vasc Biol. 2006;26:242–249. doi: 10.1161/01.ATV.0000201071.49029.17. [DOI] [PubMed] [Google Scholar]
- 27.Kamari Y, Werman-Venkert R, Shaish A, Werman A, Harari A, et al. Differential role and tissue specificity of interleukin-1α gene expression in atherogenesis and lipid metabolism. Atherosclerosis. 2007;195:31–38. doi: 10.1016/j.atherosclerosis.2006.11.026. [DOI] [PubMed] [Google Scholar]
- 28.Ray KK, Camp NJ, Benett CE, Francis SE, Crossman DC. Genetic variation at the IL-1 locus is a determinant of changes in soluble endothelial factors in patients with acute coronary syndromes. Clinical Science. 2002;103:303–310. doi: 10.1042/cs1030303. [DOI] [PubMed] [Google Scholar]
- 29.Bedard K, Krause K-H. The NOX family of ROS-generating NADPH oxidases: Physiology and Pathophysiology. Physiol Rev. 2007;87:245–313. doi: 10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- 30.Kaur J, Dhaunsi GS, Turner RB. Interleukin-1 and Nitric Oxide increase NADPH oxidase activity in human coronary artery smooth muscle cells. Medical Principles and Practice. 2004;13:26–29. doi: 10.1159/000074047. [DOI] [PubMed] [Google Scholar]
- 31.Vergnes L, Phan J, Strauss M, Tafuri S, Reue K. Cholesterol and cholate components of an atherogenic diet induce distinct stages of hepatic inflammatory gene expression. J Biol Chem. 2003;278:42774–42784. doi: 10.1074/jbc.M306022200. [DOI] [PubMed] [Google Scholar]
- 32.Wilde DW, Massey KD, Walker GK, Vollmer A, Grekin RJ. High-fat diet elevates blood pressure and cerebrovascular muscle Ca(2+) current. Hypertension. 2000;35:832–837. doi: 10.1161/01.hyp.35.3.832. [DOI] [PubMed] [Google Scholar]
- 33.Morris MC, Sacks F, Rosner B. Does fish oil lower blood pressure? A meta-analysis of controlled trials. Circulation. 1993;88:523–533. doi: 10.1161/01.cir.88.2.523. [DOI] [PubMed] [Google Scholar]
- 34.Takeuchi H, Sakurai C, Noda R, Sekine S, Murano Y, et al. Antihypertensive effect and safety of dietary alpha-linolenic acid in subjects with high-normal blood pressure and mild hypertension. J Oleo Sci. 2007;56:347–360. doi: 10.5650/jos.56.347. [DOI] [PubMed] [Google Scholar]
- 35.Yang H, Kenny A. The role of fish oil in hypertension. Conn Med. 2007;71:533–538. [PubMed] [Google Scholar]
- 36.Massaro M, Habib A, Lubrano L, Del Turco S, Lazzerini G, et al. The omega-3 fatty acid docosahexaenoate attenuates endothelial cyclooxygenase-2 induction through both NADP(H) oxidase and PKC epsilon inhibition. Proc Natl Acad Sci USA. 2006;103:15184–15189. doi: 10.1073/pnas.0510086103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Chen J, Kuhlencordt PJ, Astern J, Gyurko R, Huang PL. Hypertension does not account for the accelerated atherosclerosis and development of aneurysms in male apolipoprotein e/endothelial nitric oxide synthase double knockout mice. Circulation. 2001;104:2391–2394. doi: 10.1161/hc4501.099729. [DOI] [PubMed] [Google Scholar]
- 38.Larsen CM, Faulenbach M, Vaag A, Vølund A, Ehses JA, et al. Interleukin-1-receptor antagonist in type 2 diabetes mellitus. N Engl J Med. 2007;356:1517–1526. doi: 10.1056/NEJMoa065213. [DOI] [PubMed] [Google Scholar]
- 39.Ikonomidis I, Lekakis JP, Nikolaou M, Paraslevaidis I, Andreadou I, et al. Inhibition of IL-1 by Anakinra improves vascular and LV function in patients with rheumatoid arthritis. Circulation. 2008;117:2662–2669. doi: 10.1161/CIRCULATIONAHA.107.731877. [DOI] [PubMed] [Google Scholar]
- 40.Abbate A, Salloum FN, Vecile E, Das A, Koke NH, et al. Anakinra, a recombinant human interleukin-1 receptor antagonist, inhibits apoptosis in experimental acute myocardial infarction. Circulation. 2008;117:2670–2683. doi: 10.1161/CIRCULATIONAHA.107.740233. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Expanded methods.
(0.04 MB DOC)
Additional data
(0.04 MB DOC)
Microscopic appearances around the aortic sinus of ApoE−/− mice fed chow, Western, and WHC compared with ApoE−/−/IL-1R1−/− mice. Original magnification×2.
(1.18 MB TIF)
Modulation of IL-1 signaling decreases acute phase reactant serum amyloid A (SAA) levels. SAA was elevated in Apoe−/− mice on both high fat diets, an increase that was significantly reduced in the Apoe−/−/IL-R1−/− mice on equivalent diets. (n = 9–20)
(4.80 MB TIF)
Chromosome painting of bone-marrow transplanted mice. No Y-chromosomes were seen in male mice transplanted with female bone marrow, confirming engraftment was successful. Male recipients of male bone marrow all have Y-chromosomes, as expected, as a positive control for this method.
(2.45 MB TIF)
Vascular reactivity of arterioles from Apoe−/− and Apoe−/−/IL-R1−/− mice. Intraluminal arteriolar diameter in response to increasing pressure (0–120 mmHg). *P<0.05 Apoe−/−/IL-R1−/− (n = 6) and **P<0.05 Apoe−/− WHC (n = 6) versus Apoe−/− chow (n = 4).
(0.61 MB TIF)
ROS and Nox 4 expression in endothelial cells in culture. Increased ROS are seen following IL-1β stimulation of endothelial cells isolated from Apoe−/− mice (n = 6) (a) and human coronary endothelial cells (hCAEC) (n = 6) (b). However, no increase in ROS are seen in ECs isolated from Apoe−/−/IL-1R1−/− mice (n = 6) (a). Nox 4 mRNA expression is increased in hCAEC stimulated with IL-1β (n = 5) (c).
(9.90 MB TIF)
ROS and Nox 4 expression in vascular smooth muscle cells in culture. No significant difference in ROS are seen following IL-1β stimulation of VSMCs (n = 6) (a). Nox 4 mRNA expression is decreased in VSMC stimulated with IL-1β (n = 1) (b).
(0.30 MB TIF)
ROS generation in Apoe−/− mice following feeding of a high fat diet. Mice fed WHC had significantly more ROS than those fed chow alone, with Western diet giving intermediate levels of ROS. (n = 9).
(2.79 MB TIF)
Lipid, glucose and ALT levels in ApoE−/−/IL-1R1−/− and ApoE−/− mice fed chow, Western High Cholate (WHC), and Western diets. Data represents mean+/−SEM.
(0.05 MB DOC)
Plasma levels of IL-1ra, IL-1β, IL-1α and IL-6 in ApoE−/−/IL-1R1−/− and ApoE−/− mice fed chow, Western high cholate (WHC), and Western diets, determined by ELISA. Data represents mean+/−SEM.
(0.04 MB DOC)
Potency and efficacy of vasoconstrictor response to phenylephrine in pressurised mesenteric arterioles from Apoe−/−/IL1R1−/− and Apoe−/− mice fed a Western high cholate (WHC), Western, or Apoe−/− mice fed chow diet, assessed by pD2 (negative logarithm of EC50: concentration required for half maximum response) and Emax (percentage maximum constriction). All data are means+SEM. p = ns.
(0.04 MB DOC)