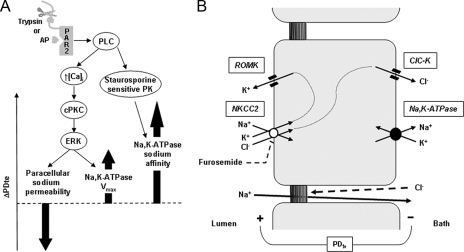
FIGURE 7.
Regulation of sodium transport by PAR2 in the rat cTAL. A, activation of basolateral PAR2, either by cleavage of its N-terminal domain by trypsin or by a synthetic peptide mimicking the ligand domain, is coupled to the activation of PLC. In turn, PLC triggers calcium signaling (↑ [Ca2+]i), which activates cPKc and induces the phosphorylation of ERK. Activation of ERK is responsible both for increased permeability of the tight junction to sodium and for enhanced Vmax of Na,K-ATPase. Because inhibition of this pathway has no significant effect on the transepithelial voltage (PDte), it is suggested that the opposite effects on PDte of increased Vmax of Na,K-ATPase and increased permeability to sodium of the paracellular pathway (thick arrows) are quantitatively similar. Activation of PLC also stimulates an unidentified staurosporine-sensitive protein kinase, which mediates an increase in the apparent affinity of Na,K-ATPase for sodium. Because inhibition of this pathway also abolishes PAR2-induced increase in PDte, the increased sodium affinity of Na,K-ATPase is likely a major actor of the change in PDte and to increased sodium reabsorption. B, basolateral Na,K-ATPase generates a sodium gradient allowing apical entry of sodium, potassium, and chloride via the furosemide-sensitive cotransporter NKCC2. Diffusive exit of potassium by apical ROMK and of chloride by basolateral ClC-K generates a transepithelial voltage (PDte) that drives paracellular reabsorption of sodium. Because the tight junctions are almost impermeable to chloride, there is no back diffusion of chloride toward the lumen.