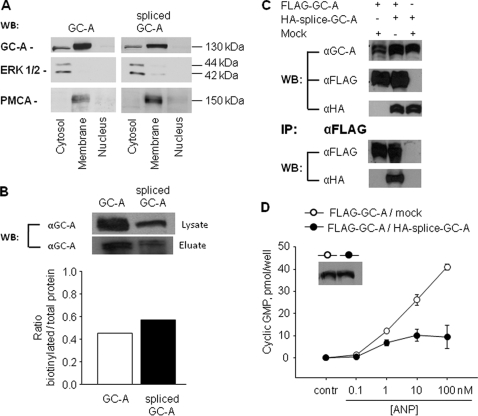
FIGURE 5.
Membrane localization of wild type and splice GC-A, heterodimer formation among GC-A isoforms and dominant negative effects of spliced GC-A on wild type GC-A. HEK 293 cells were transiently transfected with each or both GC-A isoforms. A, cell fractionation and Western blotting (WB). The detection of the ERK1/2 mitogen-activated protein kinase (MAPK) and of plasma membrane Ca2+-ATPase was used to characterize the cellular cytosolic and plasma membrane fractions, respectively. B, cell surface biotinylation. After cell surface biotinylation and purification of biotinylated protein with avidinagarose, Western blot analysis of whole-cell lysates (lysate) and biotin-labeled membrane proteins (eluate) from cells expressing wild type GC-A or spliced GC-A were performed using anti-GC-A antibodies. Similar results were observed in two independent experiments. C, coimmunoprecipitation experiments. Extracts from the cells transfected with FLAG-GC-A and pCMV5 (Mock) and the cells with FLAG-GC-A and HA-splice-GC-A were immunoprecipitated (IP) with the anti-FLAG antibody, and aliquots of cell lysates (before immunoprecipitation) as well as the immunoprecipitated proteins were analyzed by Western blot (WB) analyses with the anti-GC-A, anti-FLAG, and anti-HA antibodies. Similar results were observed in two independent experiments. D, guanylyl cyclase activity. Cells transfected with FLAG-GC-A and pCMV5 (Mock) and cells with FLAG-GC-A and HA-splice-GC-A were incubated with vehicle or ANP for 10 min, and intracellular cGMP contents were then measured (n = 3 experiments). Inset in D, Western blot analysis showing similar expression levels of total GC-A.