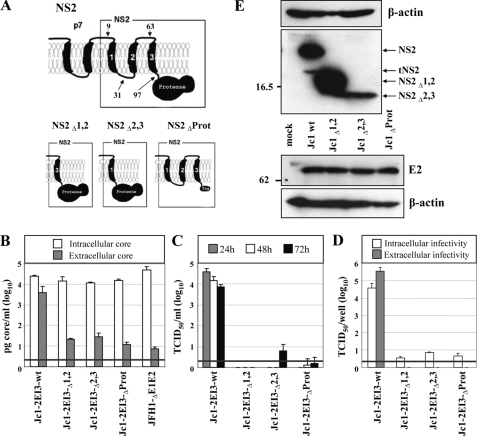
FIGURE 2.
Full-length NS2 protein is essential for production of infectious HCV particles. A, schematic diagram of the putative membrane topology of NS2 according to Refs. 17, 19 in conjunction with p7. The putative membrane topology of the NS2 deletion mutants is shown below. NS2Δ1,2 and NS2Δ2,3 lack the corresponding putative TMS (aa 10-62 and 32-96 of NS2, respectively), and in NS2ΔProt the protease domain is deleted (aa 97-217). All NS2 mutations were introduced into Jc1-2EI3. B, Huh7.5 cells were transfected with constructs specified at bottom, and the amounts of intracellular and extracellular core were determined by core-specific ELISA 48 h post-transfection. A representative result of two independent experiments with error ranges is shown. C, kinetics of release of infectious particles was measured by using TCID50 assay. D, intracellular and extracellular infectivity 48 h post-transfection as determined by TCID50 assay. Representative results of three independent experiments with error ranges are shown in C and D. E, Western blot analysis of NS2 (upper panel) and E2 proteins (lower panel) expressed in Huh7.5 cells that had been transfected with constructs specified between the panels. Cells were harvested 48 h post-transfection. Note that the tagged NS2 protein lacking the protease domain could not be detected with a tag-specific antibody (data not shown), whereas E2 amount was comparable with the other constructs. β-Actin was used as an internal loading control. The positions of molecular weight marker proteins (in kDa) are indicated at left, and HCV proteins are specified at right. tNS2, truncated NS2 protein.