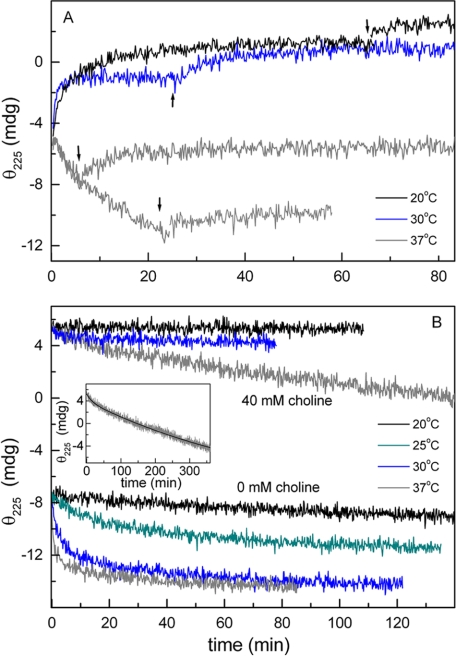
FIGURE 7.
Temperature dependence of choline binding and CBM thermal denaturation kinetics of LytC. Kinetics, monitored by measuring the protein ellipticity at 225 nm, were initiated by the addition of LytC (7.2 μm final concentration) to the cell loaded with Pi buffer (pH 6.0), supplemented, unless otherwise stated, with 40 mm choline. A shows the time course of choline binding at 20 °C (black trace), 30 °C (blue trace), and 37 °C (gray traces). At times indicated by arrows, the temperature was lowered to 4 °C, and the ellipticity changes were monitored again until re-equilibration was achieved. B shows the denaturation kinetics of the CBM of LytC in the free and choline-bound states. Measuring temperatures are indicated. The inset shows the fit of the denaturation kinetics at 40 mm choline and 37 °C in terms of two exponentials.