FIGURE 4.
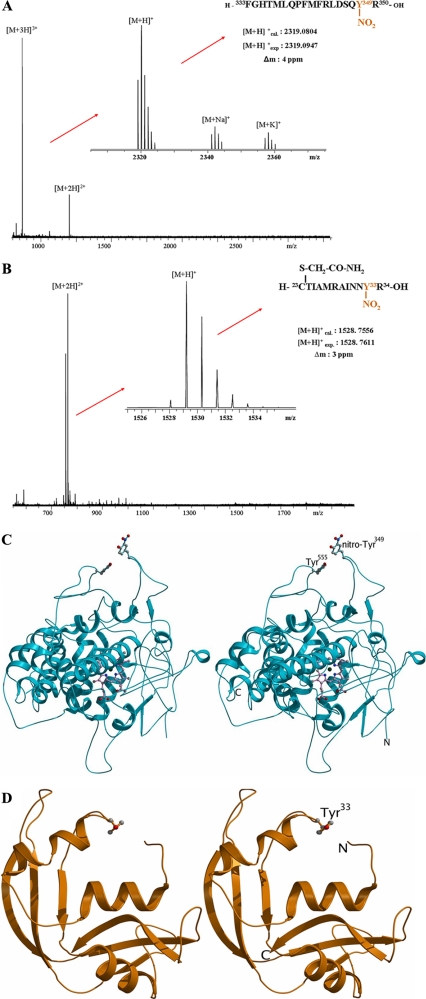
EPO carries a single, surface-exposed, 3NT residue at Tyr349, which mediates binding of EPO to positively charged surfaces. A, high resolution ESI-FT-ICR-mass spectrum of the tryptic EPO peptide 472-489-NO2. Upon deconvolution of the 3-fold charged ion, sodium and potassium adducts can be observed in addition to [M + H]+. B, high resolution ESI-FT-ICR-mass spectrum of the tryptic ECP peptide CTIAMRAINNY(NO2). Upon deconvolution of the 3-fold charged ion, sodium and potassium adducts can be observed in addition to [M + H]+. C, stereo diagram representation of an EPO model, using structural data of MPO as the target template for homology modeling. Tyr349 is represented as a ball-and-stick model. D, stereo diagram representation of ECP. Tyr33 is represented as a ball-and-stick model.