Table 2.
Room temperature palladium(II)-catalyzed methoxycarbonylation.
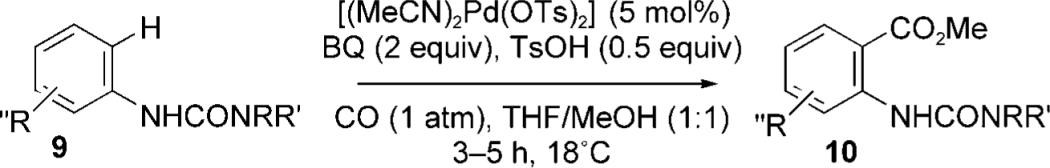 | ||||||
|---|---|---|---|---|---|---|
| Entry | 9 | R | R′ | R″ | 10 | Yield [%] |
| 1 | 9a | Me | Me | H | 10a | 88 (97)[a] |
| 2 | 9b | Et | Et | H | 10b | 76 |
| 3 | 9c | iPr | iPr | H | 10c | 55 (75)[b] |
| 4 | 9d | morpholine | H | 10d | 75 | |
| 5 | 9e | Me | H | H | 10e | 61 |
| 6 | 9f | Et | H | H | 10f | 81 |
| 7 | 9g | iPr | H | H | 10g | 90 |
| 8 | 9h | tBu | H | H | 10h | 88 |
| 9 | 9i | Bn | H | H | 10i | 85 |
| 10 | 9j | Me | Me | o-Me | 10j | 88 |
| 11 | 9k | Me | Me | m-Me | 10k | 84 |
| 12 | 9l | Me | Me | p-Me | 10l | 78 |
| 13 | 9m | Me | Me | o-MeO | 10m | 15 (40)[c] |
| 14 | 9n | Me | Me | m-MeO | 10n | 26 (60)[c] |
| 15 | 9o | Me | Me | p-MeO | 10o | 31 (80)[c] |
| 16 | 9p | Me | Me | p-CF3 | 10p | 5 (30)[c] |
| 17 | 9q | Me | Me | m-Br | 10q | 70 |
| 18 | 9r | Me | Me | p-Br | 10r | 40 |
| 19 | 9 s | Me | Me | o,p-Me | 10 s | 56 |
| 20 | 9t | Me | Me | p-CO2Me | 10t | 36 (46)[c] |
| 21 | 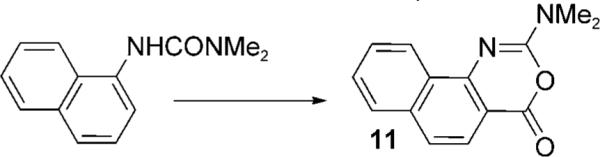 |
54 | ||||
Reactions conducted with 10 mol% precatalyst.
Reaction conducted in 100% MeOH with 3 equiv BQ.
Reactions conducted at 50 °C.
