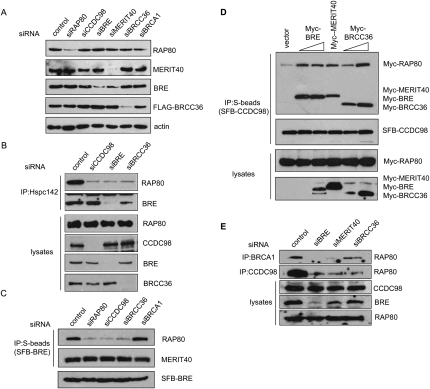
Figure 3.
MERIT40 is critical for maintaining the integrity of RAP80/CCDC98-containing protein complex. (A) U2OS cells were transfected with a scrambled control siRNA or siRNAs specific for the indicated genes. Cell lysates were prepared and immunoblotted with indicated antibodies. (B) CCDC98, BRE, and BRCC36 are required for the association of MERIT40 with RAP80. HeLa cells were transfected with control siRNA or siRNAs targeting CCDC98, BRE, or BRCC36. Cell lysates were immunoprecipitated with antibody against MERIT40, and immunoblotting experiments were carried out using antibodies as indicated. (C) CCDC98 and BRCC36 are required for the binding of BRE to RAP80, but are dispensable for BRE/MERIT40 interaction. 293T cells stably expressing SFB-BRE were transfected with control siRNA or siRNAs targeting RAP80, CCDC98, BRCC36, or BRCA1. Cell lysates were immunoprecipitated using anti-MERIT40 antibodies. Immunoblots were performed using antibodies as indicated. (D) Overexpression of BRE, MERIT40, or BRCC36 promotes RAP80/CCDC98 interaction. 293T cells were transfected with plasmids encoding SFB-tagged CCDC98 and Myc-tagged RAP80, together with empty vector or variables amount of plasmids encoding Myc-tagged BRE, MERIT40, or BRCC36 as indicated. Cell lysates were prepared, immunoprecipitated with S-protein beads, and immnoblotted with indicated antibodies. (E) Depletion of BRE, MERIT40, or BRCC36 impaired endogenous RAP80/CCDC98 or RAP80/BRCA1 interaction. HeLa cells were transfected with control siRNA or siRNAs targeting BRE, MERIT40, or BRCC36. Cell lysates were immunoprecipitated using anti-BRCA1 or anti-CCDC98 antibodies, and immunoblotting was carried out using indicated antibodies.