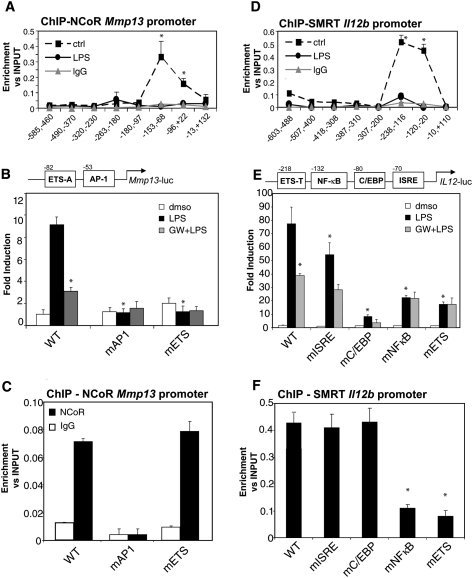
Figure 3.
NCoR is recruited to an AP-1 site in the Mmp13 promoter, and SMRT is recruited to an ETS site in the Il12b promoter. (A). NCoR occupancy of the Mmp13 proximal promoter region evaluated by ChIP assay. Primary macrophages were untreated (solid square) or treated with LPS (1 μg/mL, solid circles) for 1 h. ChIP assays were performed with an antibody against NCoR and control IgG. Immunoprecipitated DNA was analyzed by real-time PCR using primers that amplified the indicated regions of the Mmp13 promoter. (B) AP1 and ETS mutations abolished LPS induction of the Mmp13 promoter. RAW264.7 cells were transfected with luciferase reporter genes driven by the wild-type (WT) Mmp13 promoter or Mmp13 promoters with mutant AP1 or ETS sites. Twenty-four hours after transfection, cells were treated with DMSO (white bars) or with LPS (1 μg/mL) for 12 h in the absence (black bars) or presence of LXR ligand (GW3965, 1 μM, gray bars). (*) P < 0.05 versus LPS wild type. (C) The AP1 site is necessary for NCoR recruitment on the Mmp13 promoter. RAW cells were transfected with the wild-type or indicated mutant Mmp13-luciferase vectors. ChIP assays were performed with an antibody against NCoR and control IgG. Immunoprecipitated DNA was analyzed by real-time PCR using primers designed to amplify a region across the junction of the luciferase reporter coding region and the Mmp13 promoter. (*) P < 0.01 versus wild type. (D) SMRT occupancy of the Il12b proximal promoter region evaluated by ChIP assay. Primary macrophages were untreated (solid square) or treated with LPS (1 μg/mL, solid circles). ChIP assay was performed with an antibody against SMRT and control IgG. Immunoprecipitated DNA was analyzed by real-time PCR using primers that amplified the indicated regions of the Il12b proximal region. (*) P < 0.05 versus LPS. (E) LXR transrepression is impaired by ETS and NFκB mutations of the Il12b promoter. RAW264.7 cells were transfected with luciferase reporter genes driven by the wild-type (WT) Il12b promoter or Il12b promoters in which the ETS, NFκB, C/EBP, or ISRE sites were mutated. Twenty-four hours after transfection, cells were treated with DMSO (white bars) or with LPS (1 μg/mL) for 12 h in the absence (black bars) or presence of LXR ligand (GW3965, 1 μM, gray bars). (*) P < 0.05 versus LPS wild type. (F) The κB and ETS sites are necessary for SMRT recruitment to the Il12b promoter. RAW cells were transfected with the indicated wild-type or mutant Il12b-promoter/luciferase vectors, and ChIP assays were performed with an antibody against SMRT and control IgG. Immunoprecipitated DNA was analyzed by real-time PCR using primers designed to amplify a region between the luciferase and the proximal promoter region. (*) P < 0.05 versus wild type. Results are expressed as average of three independent experiments. Error bars represent standard deviations.