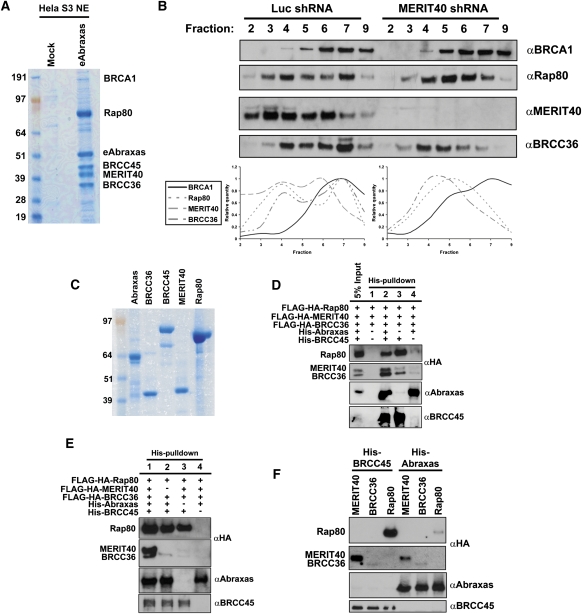
Figure 6.
Biochemical reconstitution of the Rap80 complex in vitro. (A) Coomassie-stained gel representing eAbraxas complexes after consecutive Flag and HA affinity-purification steps from HeLa S3 nuclear extracts (NE). The predominant interacting proteins are readily observed in strong Coomassie-stained bands for Rap80, eAbraxas, BRCC45, MERIT40, and BRCC36. BRCA1 was detected substoichiometrically as an Abraxas-associated protein. (B) HeLa S3 nuclear extracts derived from cells expressing shRNA to either luciferase (luc) or MERIT40 were centrifuged through a 5%–22.5% sucrose gradient, and individual fractions were probed by IB as indicated. MERIT40 depletion shifts peak protein levels of Rap80 and BRCC36 to lower fractions. Quantification of bands was performed using ImageJ software from the NIH. (C) Coomassie-stained gel representing purified proteins used for in vitro binding assays. Recombinant Abraxas and BRCC45 are His-NusA- and His-ZZ-tagged, respectively; were bacterially derived; and are estimated to be ∼90% pure. Recombinant BRCC36, MERIT40, and Rap80 derived from baculovirus-infected Sf9 cells are Flag-HA-tagged and are estimated to be ∼95% pure. (D,E) IB of in vitro binding assays was performed as indicated. BRCC36, MERIT40, and Rap80 were baculovirally expressed and mixed in equal molar concentrations with both Abraxas and BRCC45 or combinations thereof. HisPur Cobalt resin was used for coprecipitation of recombinant proteins. (F) IB of in vitro binding assays was performed as indicated. His-tagged BRCC45 or His-tagged Abraxas were mixed in equal molar concentration with Rap80, MERIT40, or BRCC36 individually and coprecipitated using HisPur Cobalt resin.