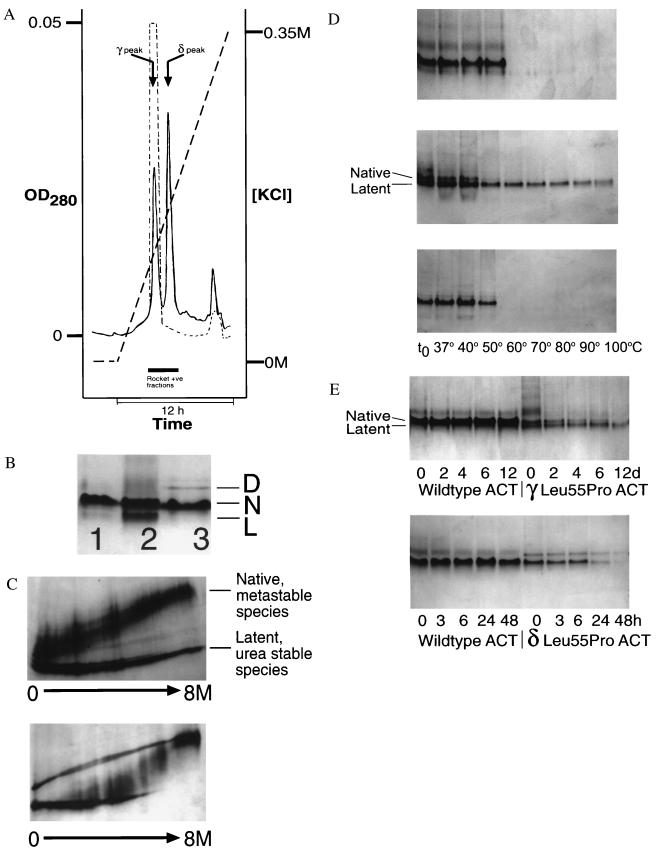
Figure 2.
(A) Elution profile of Leu-55–Pro α1-antichymotrypsin from heparin-Sepharose showing a peak (γ) that elutes at the same KCl concentration as wild-type protein (dashed line) and a higher affinity peak (δ). The peak that elutes near the end of the gradient contains dimeric species. (B) 7.5–15% (wt/vol) nondenaturing PAGE of recombinant wild-type α1-antichymotrypsin (lane 1), recombinant γ Leu-55–Pro α1-antichymotrypsin (lane 2), recombinant δ Leu-55–Pro α1-antichymotrypsin (lane 3). Lanes 1 and 3 contain 7.5 μg protein and lane 2 contains 20 μg to give similar band intensities. γ Leu-55–Pro α1-antichymotrypsin migrated as two bands with the electrophoretic mobility of native (N) and latent (L) α1-antichymotrypsin. δ Leu-55–Pro α1-antichymotrypsin had a similar mobility to recombinant wild-type α1-antichymotrypsin with a high molecular mass dimer (D). (C) γ Leu-55–Pro α1-antichymotrypsin (Upper) showed native and latent unfolding transitions on transverse urea gradient PAGE whereas the δ protein (Lower) unfolded with an intermediate profile at approximately 4 M urea. The left of each gel represents 0 M urea and the right 8 M urea. Each gel was loaded with 20 μg protein, which was visualized by silver staining. (d) 7.5–15% (wt/vol) nondenaturing PAGE assessing the thermal stability of wild-type (Top), γ Leu-55–Pro α1-antichymotrypsin (Middle), and δ Leu-55–Pro α1-antichymotrypsin (Bottom). Samples were incubated at 0.3 mg/ml for 30 min at the temperatures indicated, and 7.5 μg protein was loaded in each lane. (E) The native conformation of γ (Upper) and δ (Lower) Leu-55–Pro α1-antichymotrypsin were less stable (as evidenced by the loss of bands on nondenaturing PAGE) than the wild-type protein when incubated under physiological conditions. The samples were incubated at 0.25 mg/ml in 50 mM Tris, 50 mM KCl, pH 7.4 at 41°C for the times indicated and then assessed by 7.5–15% (wt/vol) nondenaturing PAGE. All lanes contain 7.5 μg protein.