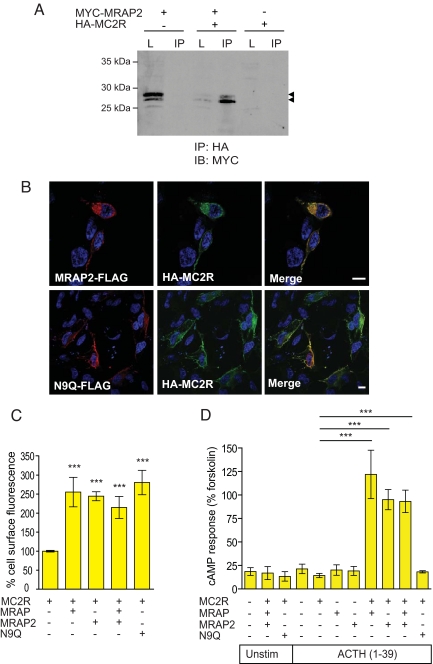
Fig. 2.
N-terminally glycosylated MRAP2 enables the functional expression of MC2R. (A) Co-IP showing interaction between HA-MC2R and MYC-MRAP2. L, lysate; IB, immunoblotting. Arrowheads indicate MRAP2 bands. (B) HA-MC2R colocalizes with MRAP2-FLAG at the cell surface, as seen by immunofluorescence. The deglycosylation MRAP2 mutant N9Q-FLAG also colocalizes with HA-MC2R at the cell surface. (Scale bars, 10 μm.) (C) MRAP2 allows trafficking of HA-MC2R to the cell surface. Using a fluorescent cell surface assay, HA-MC2R is detected at the cell surface when coexpressed with MRAP2, MRAP, MRAP+MRAP2, and N9Q. There is no significant difference between HA-MC2R trafficking with either MRAP or MRAP2. N9Q also enables HA-MC2R cell-surface trafficking. Error bars represent SEM. The x axis represents relative intensity normalized to MC2R alone (100%). (D) N-terminally glycosylated MRAP2 enables HA-MC2R responsiveness to ACTH using a cAMP accumulation assay. MC2R was expressed alone or with MRAP2, MRAP, MRAP+MRAP2, and N9Q in HEK293T cells. Cells were either unstimulated (Unstim) or stimulated with 10−6 M ACTH(1–39) or 10−5 M forskolin. MC2R is responsive to ACTH when coexpressed with MRAP2. There is no significant difference to response generated when MC2R is coexpressed with MRAP or both MRAP and MRAP2. With the MRAP2 glycosylation mutant, N9Q, MC2R shows no response to ACTH. ***, P < 0.0001.