Figure 2. In vivo and in vitro hydroxylation of Ago2.
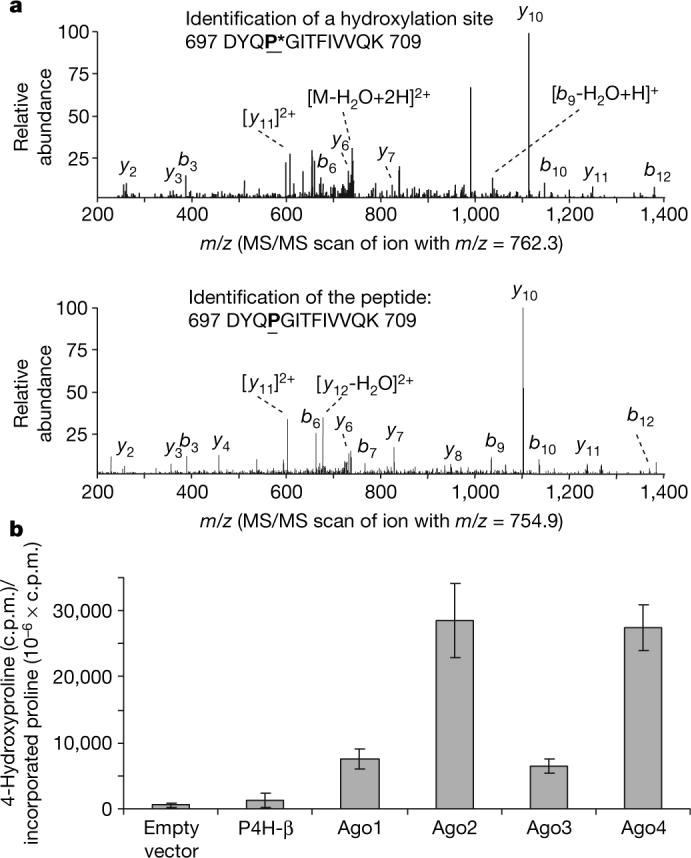
a, Endogenous Ago2 is hydroxylated at proline 700. Endogenous Ago2 was immunoprecipitated with a polyclonal anti-Ago2 antibody and analysed by LC–MS/MS. The MS/MS scanned hydroxylated peptides with ion m/z 762.3 (upper panel) and ion m/z 754.9 (lower panel). b, In vitro hydroxylation of Ago proteins. pcDNA3–Flag-HA-Ago1−4 were in vitro translated in the presence of L-[2,3,4,5-3H]proline. The in vitro translated lysates were incubated with purified recombinant C-P4H(I) enzyme and the reaction cofactors. The amount of 4-hydroxy[3H]proline formed in control, P4H-β and Ago 1−4 was determined as described in Methods. Data are presented as the mean ± standard deviation for three independent experiments.
