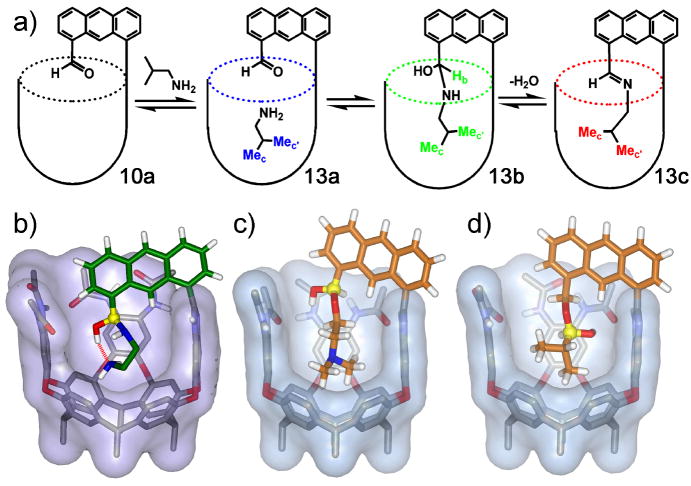
Figure 6.
a) The stabilization of the hemiaminal formed from addition of isobutylamine to cavitand 10a; b) Minimized structure of the hemiaminal formed by addition of ethylenediamine to 10a; the hydrogen bond between the terminal amine and hemiaminal OH is shown; c) Minimized structure of the hemiacetal formed by addition of N,N’-dimethylaminoethanol to 10a; the newly formed OH group is located near the amides and is stabilized by a factor of 5800; d) Minimized structure of the hemiacetal formed by addition of isopropionaldehyde to 10b; the newly formed OH group is located near the aromatic walls and is only stabilized by a factor of 140.