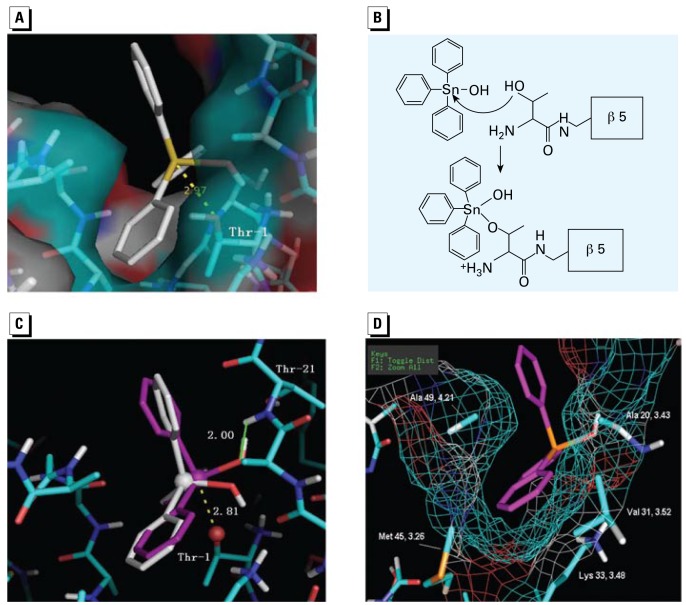
Figure 2.
Docking studies for the interaction of TPT and the proteasome β5 subunit. (A) The docking model of TPT-OH with a tetrahedral conformation in the proteasomal β5 CT site; TPT-OH is represented by a stick structure. (B) A hypothetical mechanism for the interaction between TPT-OH and the β5 Thr-1 residue. (C) Comparison of TPT-OH with tetrahedral conformation (white) and trigonal bipyramidal conformation (pink) in the β5 site. The three phenyl rings, one OH legend, and Oγ on Thr-1 form the trigonal bi pyramidal conformation; the Sn on TPT and Oγ of Thr-1 are shown as spheres. (D) The hydrophobic interaction between the phenyl ring of TPT and the hydrophobic side chains in the S1 pocket. The mesh represents the surface of the S1 pocket.