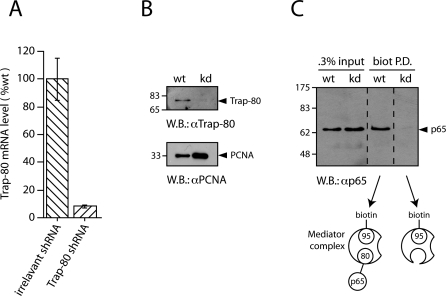
Figure 1. Removal of Trap-80 Disrupts the Interaction of p65 with Mediator.
(A) Knock-down of Trap-80 mRNA by RNA interference. Expression level of Trap-80 mRNA in 3T3 fibroblasts expressing an shRNA targeting Trap-80, shown as a percentage of the level in control cells (expressing an irrelevant shRNA, hereafter referred to as wild type). mRNA levels were measured by quantitative PCR and normalized with respect to Tbp. Error bars indicate standard errors; the results presented here are representative of more than ten experiments.
(B) Knock-down of Trap-80 protein levels. Nuclear extracts were prepared from wild-type (wt) or Trap-80 knock-down (kd) fibroblasts, and endogenous Trap-80 (upper panel) or PCNA (as a loading control, lower panel) was detected by western blotting.
(C) Co-precipitation of p65 and Trap-95 depends on the presence of Trap-80. Wild-type and Trap-80 knock-down fibroblasts were simultaneously transduced with retroviruses expressing biotin-tagged Trap-95 and the BirA biotin ligase, and nuclear extracts were prepared after stimulation with TNF-α. The Mediator complex, containing biotinylated Trap-95, was pulled down using streptavidin beads (biot P.D.), and any associated p65 was detected by western blotting. Pull-down using in vivo biotinylation is our method of choice for analysis of proteins bound to the endogenous Mediator complex, due to the extremely high affinity of the streptavidin-biotin interaction. The levels of biotin-tagged Trap-95 were similar in both cell types, as measured by the level of co-expressed Tomato fluorescent protein (Figure S3). Dotted lines indicate where nonrelevant lanes have been cropped from the figure.