Abstract
WHAT IS ALREADY KNOWN ABOUT THIS SUBJECT.
The effects of statins may be beneficial to patients with chronic heart failure.
However, one question that has not yet been answered and that may clarify the role of statins in chronic heart failure (CHF) is whether statins prevent the development of CHF in patients with a low risk of cardiovascular disease (CVD)?
WHAT THIS PAPER ADDS.
The study results demonstrate that, in primary prevention, adherence to statins has a positive impact on CHF.
This study provides evidence of the potential role of statins in CHF.
AIMS
Statins are effective in the prevention of an atherosclerotic event, e.g. coronary artery disease and cerebrovascular disease. Patients at high risk of cardiovascular disease (CVD), such as chronic heart failure (CHF), might benefit from the effects of statin therapy. However, one question that has not yet been answered and that may clarify the role of statins in CHF is whether statins prevent the development of CHF in patients with a low risk of CVD. Our aim was to evaluate the impact of adherence to statins on the incidence of CHF.
METHODS
A cohort of 111 481 patients was reconstructed using the Régie de l'Assurance Maladie du Québec databases. Patients were eligible if they were between 45 and 85 years old, without CVD, and newly treated with statins between 1999 and 2004. A nested case–control design was used to study CHF. Every case of CHF was matched for age and duration of follow-up in up to 15 randomly selected controls. The adherence level was measured by calculating the medication possession ratio. Rate ratios (RR) of CHF were estimated by conditional logistic regression adjusting for several covariables.
RESULTS
The mean patient age was 63 years, 49% had hypertension, 21% had diabetes and 41% were male. A high level of adherence to statins was associated with a reduction of CHF (RR 0.81; 0.71, 0.91). The risks associated with CHF were the development of CVD during follow-up, being a social-aid recipient, and suffering from hypertension, diabetes mellitus, or having a higher chronic disease score.
CONCLUSION
Our study indicates that better adherence to statins is associated with a reduced risk of CHF.
Keywords: adherence to medical regimen, heart failure, statins
Introduction
The treatment of cardiovascular disease (CVD) represents the highest healthcare costs in most industrialized countries [1]. The most common cause of chronic heart failure (CHF) is no longer hypertension or valvular heart disease, but rather coronary artery disease (CAD) [2–4], which is also the leading cause of left ventricular dysfunction [4]. Approximately 50% of incident cases of CHF in subjects <75 years old are related to CAD [5, 6].
Statins are effective for primary and secondary prevention of atherosclerotic events, such as CAD and a cerebrovascular disease [7]. The alteration of lipoprotein levels is the major reason for this benefit, but we could not exclude other nonlipid-related effects. Patients at high risk of CVD, such as those with CHF, may particularly benefit from reduced levels of inflammatory factors and detrimental cytokines, improved endothelial function, and stabilized coronary plaque [8–11]. However, there are potential detrimental effects of statin therapy in CHF such as reductions in coenzyme Q10 [12] and selenoprotein [13] levels, which could have adverse effects on myocyte structure and function, and decreased ability of lipoproteins to bind endotoxins, leading to excessive inflammation [14]. Nevertheless, the possible beneficial effects of statins may outweigh its potential risks [12–14].
A systematic review of observational studies of patients hospitalized for CHF with advanced CHF or impaired systolic function has suggested that the use of statins is related to reduce mortality and morbidity [15]. Moreover, small clinical trials have yielded mixed results for intermediate markers related to inflammatory components or left ventricular function [16–19].
Considering the high morbidity and mortality rates in persons with CHF, some might recommend statin use. In fact, it is impossible to recommend routine statin use for all patients with CHF irrespective of the aetiology. A question that remains to be answered and that may help to clarify the role of statins in CHF is whether statins prevent CHF development in a population with a lower risk of CVD. The aim of this study was to examine the association of statin adherence in primary prevention with the risk of developing CHF.
Methods
Data sources
This population-based study used the databases of the Réie de l'Assurance Maladie du Quéec (RAMQ) and MedEcho, which administers public healthcare insurance programmes in Quebec, Canada. The RAMQ databases contain three types of files. The demographic file lists age, gender, postal code and year of death for all registered individuals. The medical-services file comprises claims for all inpatient or ambulatory medical services and includes data such as the nature of the medical procedure, date and site (office, emergency room, hospital) of the procedure, and the diagnostic code [20]. The diagnosis is coded in accordance with the International Classification of Disease (ICD-9). Procedural codes follow the Canadian classification of diagnostic, therapeutic and surgical procedures [20, 21]. These codes are linked to the physician payment and are carefully audited. The pharmaceutical file contains data on all prescriptions for covered drugs prescribed to patients living in the community whose medications are insured by RAMQ. The file includes the name, dose and quantity of the drug, the date, and the duration of therapy as indicated by the pharmacist. The Med-Echo database contains data on acute care hospitalizations, such as date of admission, length of stay as well as primary and secondary diagnoses. All these files also contain the individual's health insurance number, which is the link between them.
The first two of the RAMQ's databases include the entire population of Quebec. The pharmaceutical file covers all residents insured under the Public Prescription Drug Insurance Plan, which covers about 43% of all residents [22] between 1998 and 2005, of which 94% of Quebec citizens aged ≥65 years were covered by the plan [23]. Each of the computerized files contains the individual's health insurance number, which serves as a link between the files. The pharmaceutical file has been validated for research and used in pharmacoepidemiological research studies [24]. The prescription claims database in Quebec is an accurate means of determining the drugs dispensed to individuals. Validity studies have been also performed for medical services claims from the RAMQ databases [25–27].
In the present study, we used the diagnostic codes identifying episodes of hospitalization for CHF by using the coding of The Epidemiology, Practice, Outcome and Costs of Heart Failure (EPOCH) study cohort [28], and the diagnosis of CHF was confirmed with the Framingham Study clinical criteria [29]. From a sample of the EPOCH cohort members who had a primary discharge diagnosis of heart failure, 93.6% had confirmed heart failure using the Framingham criteria. In addition to the diagnostic codes identifying episodes of hospitalization for CHF, we also included CHF events by adding medication markers.
Cohort study
We selected a cohort of patients who had started treatment with atorvastatin, fluvastatin, lovastatin, pravastatin, simvastatin or rosuvastatin between 1 January 1999 and 31 December 2004, but who had not taken any lipid-lowering drugs in the year preceding the first prescription. The date of the first prescription of a statin was defined as the cohort entry date. Patients had to be between 45 and 85 years old and to have been insured for their drugs by the RAMQ for at least 2 years prior to entry into the cohort.
In order to be eligible, subjects must not have had any indication of CVD as evidenced by the absence of a diagnosis or medical procedure in the previous 5 years and any drug marker in the 2 years prior to the cohort entry date. Patients had to be free of any CVD marker such as (i) CAD: diagnosis of myocardial infarction or angina (ICD-9 codes 410–414), a medical procedure, i.e. coronary artery bypass grafting, angiography, or angioplasty or stent, or use of nitrate, including nitroglycerin; (ii) cerebrovascular disease: diagnosis (430–438) or medical procedures or use of nimodipine; (iii) peripheral artery disease (PAD): diagnosis of peripheral vascular disease (440–447), medical procedure of noncoronary angioplasty or use of pentoxifylline; (iv) CHF: diagnosis of CHF (398.91, 402.01, 402.11, 402.91, 428.0, 428.1 and 428.9) or the use of furosemide alone or with digoxin, angiotensin converting enzyme (ACE) inhibitors, spironolactone or β-blockers; (v) arrhythmia: diagnosis (427), a medical procedure using a pacemaker or the use of drugs for cardiac arrhythmias; (vi) valvular heart disease; (vii) renal disease: diagnosis of renal disease (580-589), haemodialysis, peritoneal dialysis or drug markers; or (viii) hepatic disease: diagnosis of hepatic disease (570). The RAMQ drug database was used also to exclude patients who received other drugs such as antiplatelets (excluding low-dose aspirin) or anticoagulants during the 2 years preceding the cohort entry.
The final study cohort included 111 481 subjects who were followed from the date of the first claim for a statin until the first CHF event or 30 June 2005. Subjects were censored if they switched to another class of lipid-lowering drugs (such as fibrates, etc.), loss of coverage by RAMQ drug plan, renal insufficiency, hepatic disease, or died. Subjects were followed for a minimum of 6 months to a maximum of 6.5 years. The total death rate was assessed in the cohort.
Nested case–control study
The nested case–control was used to estimate the rate ratio (RR) of the first CHF associated with adherence level to statins. A CHF event was defined as the index date by a composite end-point as defined by a diagnosis (ICD-9 codes 398.91, 402.01, 402.11, 402.91, 428.0, 428.1 and 428.9) or the use of furosemide alone or with digoxin, ACE inhibitors, spironolactone or β-blockers. All cases of CHF were identified and up to 15 controls were randomly selected from the risk set for each case using density sampling, e.g. by matching on age and the same follow-up time [30].
Assessment of exposure
Within each risk set, adherence level was measured by calculating the medication possession ratio (MPR), which was defined as the number of days' supply of medication received divided by the length of follow-up [31, 32]. The patients' adherence level was calculated from the start of follow-up to the time of a CHF event. In the case of the controls, the adherence level was calculated from the start of follow-up to the time of selection. MPR was evaluated as categorical variable; for example, ≥80% of the prescribed doses, 60–79%, 40–59%, 20–39% and 1–19% (reference group) were used [33]. Based upon the data in the literature [34–36], the equivalent in lowering low-density lipoprotein (LDL)-cholesterol levels was estimated by using an equivalent dose of simvastatin.
Confounding variables
The social assistance status was identified from data in the beneficiary's file in the RAMQ database at the cohort entry date. During follow-up, the fact of developing a CAD, cerebrovascular disease, PAD or other CVD events (suffering either from cardiac arrhythmia, pulmonary circulation problems or miscellaneous heart illnesses or being known for taking anticoagulant drugs) was also included in the model as covariable. Diabetes and hypertension were identified in the year prior to cohort entry and during follow-up, and these were defined as follows: diabetes by ICD-9 code 250 or by the use of insulin or an antidiabetic agent, and hypertension by essential hypertension ICD-9 code 401 or by the use of thiazides, ACE inhibitors without furosemide, calcium channel blockers, or β-blockers without other markers of CAD. Patients with diabetes or hypertension diagnosed in the year preceding the index date were considered as newly diagnosed. For the other patients, the use of antidiabetic or antihypertensive agents in the year before the index date was dichotomized into two levels: high adherence, indicated by having filled >80% of the prescribed doses, and low adherence, by having filled <80%. Patients who were diagnosed with diabetes mellitus or hypertension but never treated were defined as such. The reference categories comprised subjects without hypertension and subjects without diabetes, respectively. Dichotomized variables were also used for respiratory diseases, use of antidepressant or anxiolytic agents, and a modified chronic disease score (level ≥4 or <4) [37]; and the exposure was identified in the year preceding the index date.
Statistical analysis
In multivariable analysis, a conditional logistic regression model was constructed to evaluate the association between statin adherence and CHF. When studying an exposure that varies with time, as was the case in our study (adherence to statins), an additional level of complexity is introduced by the need to account for time-dependent exposure in the analysis; and this can be accomplished by cohort analysis using Cox regression including time-dependent covariates. Alternatively, a nested case–control approach can be used providing the exposure and covariates information for controls reflecting values corresponding to the time of selection of their respective case. Nested case–control analyses have been found to yield results that were similar to results of Cox regression on the full cohort when studying time-dependent exposures, with the advantage of superior computational efficiency with the conditional logistic regression, given that only a sample of all possible controls is included in the risk set of each case [38].
Unselected multivariable models were constructed to adjust maximally for confounding and included all variables described in the Covariables section. The possible effect of time was taken into account by stratifying the analysis by the time of case occurrence: cases occurring in the first year of follow-up and those after 1 year of follow-up. The crude and adjusted RRs for CHF were determined through conditional logistic regression. Subgroup analyses were created on the basis of age groups, or comorbidities, such as diabetes or hypertension.
The robustness of our findings with regard to potential biases introduced by unmeasured confounders was evaluated by using a Monte-Carlo approach proposed by Greenland [39, 40]. We created several scenarios with different risk factors between the confounder and CHF [41–43]. For each scenario, the prevalence of the unmeasured confounder across adherence categories was changed. Using this analysis, we determined how the RR changes after adjusting for the unmeasured confounder. Residuals from regression models were assessed for violations of the assumptions of multicollinearity or deviance [44, 45]. All analyses were performed using Statistical Analysis System Software (version 9; SAS Institute, Cary, NC, USA). All analyses with 95% confidence intervals (CI) are presented.
Ethical considerations
No identifiers related to patients or physicians were provided to the researchers. The Research and Ethics Committee of the University of Montreal approved the study.
Results
Patient characteristics
A flow chart of the selected cohort is shown in Figure 1. Of the 111 481 identified patients, the mainly prescribed statins were atorvastatin, pravastatin and simvastatin (Table 1). Comparison of baseline demographic and clinical characteristics revealed no major differences across the statins. Regarding the distribution of daily doses, we found that in most cases lower doses of statins were prescribed.
Figure 1.
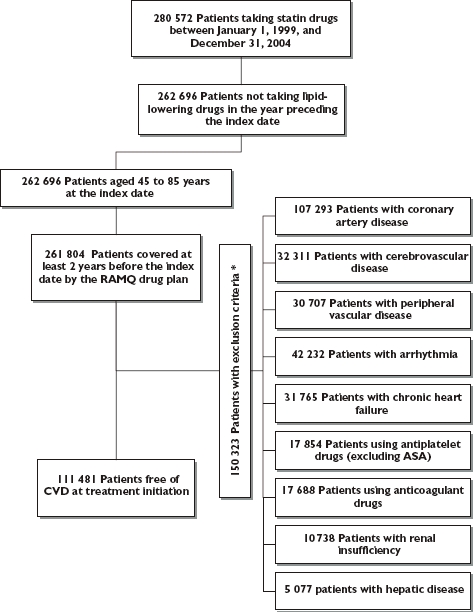
Flow chart of inclusion and exclusion criteria* *Exclusion criteria were assessed in the 2 years preceding the index date for the medication and in the 5 years preceding the cohort entry for hospitalizations, diagnosis, or medical procedures
Table 1.
Characteristics of patients initiating a new statin treatment in Quebec RAMQ database in 1999–2004
| Atorvastatin | Fluvastatin | Lovastatin | Pravastatin | Rosuvastatin | Simvastatin | |
|---|---|---|---|---|---|---|
| Number of patients | 70 269 | 2207 | 812 | 13 660 | 7782 | 16 747 |
| Follow-up time (continuous) | 1 055 (±606) | 1514 (±614) | 1498 (±644) | 1 322 (±642) | 427 (±135) | 1 157 (±603) |
| Mean dose (mg) | 13 (±6) | 26 (±10) | 21 (±6) | 20 (±8) | 11 (±3) | 17 (±9) |
| Mean age (continuous)† | 63 (±10) | 63 (±10) | 63 (±10) | 64 (±10) | 63 (±10) | 64 (±10) |
| Sex (% male) | 42 | 35 | 34 | 37 | 45 | 41 |
| Social assistance (%)† | 16 | 18 | 18 | 15 | 14 | 14 |
| Diabetes mellitus (%)‡ | 21 | 15 | 15 | 19 | 21 | 23 |
| Hypertension (%)‡ | 49 | 46 | 43 | 48 | 52 | 52 |
| Respiratory diseases (%)‡ | 10 | 9 | 9 | 10 | 10 | 10 |
| Antidepressant drugs (%)‡ | 9 | 8 | 10 | 9 | 10 | 9 |
| Anxiolytic drugs (%)‡ | 25 | 27 | 29 | 26 | 24 | 26 |
| Dose distribution† | ||||||
| 5 mg | 1% | 0% | 0% | 0% | 1% | 6% |
| 10 mg | 78% | 0% | 7% | 16% | 95% | 43% |
| 20 mg | 19% | 62% | 87% | 70% | 4% | 44% |
| 40 mg | 1% | 38% | 6% | 8% | 0% | 7% |
| 80 mg | 0% | 0% | 0% | 0% | 0% | 0% |
At the treatment initiation.
ICD-9 or pharmacological treatment in the year prior to the cohort entry.
In the full cohort, 41% were men, 16% welfare recipients, 54% had hypertension and 26% had diabetes. During follow-up, 3.8% had had only CHF (1.3/100 person-years), 6.4% of them only CAD (2.2/100 person-years), 1.8% had only cerebrovascular disease (0.6/100 person-years), 1.5% presented only a PAD (0.5/100 person-years), 4.8% had other CVD events (1.6/100 person-years); but 13% had more than two CVD events (4.4/100 person-years). The percentage of death during follow-up was 2.0% (0.7 per 100 person-years).
The proportion of men, social assistance, patients with diabetes, hypertension or respiratory diseases, users of antidepressant or anxiolytic agents was statistically higher among the cases (Table 2). In addition, cases had experienced more CVD events during follow-up than controls, as well as presenting a higher chronic disease score.
Table 2.
Characteristics of patients with chronic heart failure and their matched controls
| Cases occurring in the first year of follow-up and their controls | Cases occurring after 1 year of follow-up and their controls | |||
|---|---|---|---|---|
| Cases | Controls | Cases | Controls | |
| Numbers | 1369 | 20 462 | 2940 | 43 661 |
| Age (continuous)† | 68 (±9) | 68 (±9) | 67 (±9) | 67 (±9) |
| Mean equivalent dose (mg)‡ | 25 | 23 | 23 | 21 |
| Statin adherence§ | ||||
| 1–19% | 5% | 5% | 13% | 15% |
| 20–39% | 5% | 6% | 9% | 9% |
| 40–59% | 8% | 6% | 10% | 9% |
| 60–79% | 8% | 9% | 13% | 13% |
| ≥80% | 74% | 74% | 55% | 54% |
| Sex (% male) | 40 | 38 | 39 | 36 |
| Social assistance† | 14% | 9% | 15% | 10% |
| Having a CAD during follow-up†† | 14% | 2% | 14% | 7% |
| Having a cerebrovascular disease during follow-up¶ | 2% | 1% | 3% | 2% |
| Having a PAD during follow-up** | 0.3% | 0.6% | 1.7% | 1.7% |
| Having other CVD events during follow-up‡‡ | 8% | 1% | 11% | 4% |
| Having ≥2 CVD events | 18% | 0.8% | 30% | 6% |
| Diabetes§§ | 50% | 29% | 50% | 31% |
| Diagnosed and nontreated§§ | 5% | 6% | 6% | 7% |
| Newly diagnosed for diabetes mellitus§§ | 10% | 6% | 2% | 2% |
| Antidiabetic agents adherence <80%§§,¶¶ | 8% | 4% | 12% | 6% |
| Antidiabetic agent adherence ≥80%§§,¶¶ | 27% | 13% | 30% | 16% |
| Hypertension§§ | 75% | 62% | 85% | 70% |
| Diagnosed and nontreated§§ | 3% | 5% | 3% | 7% |
| New diagnosed for hypertension§§ | 16% | 14% | 5% | 4% |
| Antihypertensive agent adherence <80%§§,¶¶ | 11% | 7% | 18% | 13% |
| Antihypertensive agent adherence ≥80%§§,¶¶ | 45% | 36% | 59% | 46% |
| Respiratory diseases§§ | 25% | 11% | 33% | 18% |
| Antidepressant drugs§§ | 13% | 9% | 18% | 13% |
| Anxiolytic drugs§§ | 36% | 29% | 46% | 37% |
| Chronic disease score (≥4) | 17% | 9% | 25% | 11% |
At treatment initiation.
Statins equivalent in simvastatin dose during follow-up simvastatin 20 mg = lovastatin 40 mg = pravastatin 40 mg = fluvastatin 80 mg = atorvastatin 10 mg = rosuvastatin 5 mg.
Proportion of days covered (%).
Diagnosis of cerebrovascular disease: (ICD-9 codes 430–438) or medical procedures.
Diagnosis of peripheral artery disease (PAD): diagnosis (ICD-9 codes 440–447), medical procedure of noncoronary angioplasty, or use of pentoxifylline).
Diagnosis of coronary artery disease (CAD): myocardial infarction or angina (ICD-9: 410–414), a medical procedure, i.e. coronary artery bypass grafting, angiography, or angioplasty, use of nitrate, including nitroglycerin.
Diagnosis of other cardiovascular disease (CVD): arrhythmia: diagnosis (ICD-9 code 427), a medical procedure using a pacemaker and the use of drugs for cardiac arrhythmias (amiodarone, digoxin, quinidine, disopyramide, flecainamide, mexiletine, procainamide, propafenone, or sotalol); or valvular heart disease; or anticoagulants.
ICD-9 or pharmacological treatment.
Proportion of days covered (%) in the year before the index date.
Impact of adherence level on CHF and the risk of CHF
The mean high adherence level of statins was approximately 74% and 54% during the first year and after 1 year of follow-up, respectively. As shown in Table 3, in the multivariate model the CHF rate decreased by 19% among the group with a high adherence level compared with the reference group (RR 0.81; 0.71, 0.91). When the analysis was stratified by the time of case presentation, we found that high adherence seemed to have had an impact on CHF in the year following the initiation of statins. In the multivariate model, social assistance, diabetes, hypertension, respiratory disease and anxiolytic drug use increased the risk of CHF. Developing a CAD, cerebrovascular disease, PAD or other CVD events during follow-up significantly increased the risk of CHF from 2.0 to 9.0; and those estimates increased even more in the first year following the development of CVD. Again, subjects having experienced more than one CVD event received an important risk of CHF. Finally, having a high chronic disease score also increased the risk of CHF.
Table 3.
Rate ratio of chronic heart failure
| Rate ratio (95% CI) | ||||
|---|---|---|---|---|
| Cases occurring in the first year of follow-up and their controls | Cases occurring after 1 year of follow-up and their controls | |||
| Crude | Adjusted | Crude | Adjusted | |
| Statin adherenceठ| ||||
| 1–19% | Reference | Reference | Reference | Reference |
| 20–39% | 0.74 (0.52, 1.05) | 0.70 (0.46, 1.03) | 1.04 (0.88, 1.22) | 0.90 (0.76, 1.07) |
| 40–59% | 1.19 (0.87, 1.63) | 1.01 (0.70, 1.45) | 1.11 (0.95, 1.30) | 0.91 (0.77, 1.08) |
| 60–79% | 0.84 (0.61, 1.15) | 0.62 (0.43, 0.99) | 1.06 (0.92, 1.23) | 0.88 (0.75, 1.03) |
| ≥80% | 0.95 (0.73, 1.23) | 0.72 (0.53, 0.98) | 1.08 (0.96, 1.21) | 0.81 (0.71, 0.91) |
| Sex (male vs. female) | 1.10 (0.98, 1.23) | 0.97 (0.85, 1.10) | 1.13 (1.04, 1.22) | 1.05 (0.96, 1.14) |
| Social assistance† (yes vs. no) | 1.84 (1.52, 2.23) | 1.58 (1.27, 1.97) | 1.83 (1.61, 2.08) | 1.28 (1.11, 1.48) |
| Having a CAD during follow-up†† | 7.98 (6.63, 9.62) | 11.76 (9.52, 14.53) | 2.20 (1.97, 2.47) | 3.94 (3.46, 4.49) |
| Having cerebrovascular disease during follow-up¶ | 2.33 (1.45, 3.76) | 4.15 (2.50, 6.89) | 1.21 (0.96, 1.53) | 2.39 (1.86, 3.05) |
| Having a PAD during follow-up** | 0.52 (0.19, 1.41) | 0.92 (0.33, 2.54) | 1.04 (0.78, 1.38) | 2.02 (1.50, 2.72) |
| Having an other CVD events during follow-up‡‡ | 7.12 (5.58, 9.08) | 10.83 (8.23, 14.25) | 2.62 (2.31, 2.97) | 4.48 (3.88, 5.18) |
| Having ≥2 CVD events | 29.74 (23.8, 37.1) | 45.39 (35.3, 58.33) | 7.22 (6.59, 7.92) | 9.46 (8.46, 10.58) |
| No diabetes | Reference | Reference | Reference | Reference |
| Diabetes diagnosed and nontreated §§ | 1.10 (0.85, 1.44) | 0.94 (0.70, 1.27) | 1.34 (1.15, 1.57) | 1.13 (0.95, 1.33) |
| New diagnosed for diabetes mellitus §§ | 2.08 (1.71, 2.53) | 1.98 (1.59, 2.48) | 1.88 (1.43, 2.48) | 1.40 (1.04, 1.89) |
| Antidiabetic agent adherence <80% §§,¶¶ | 2.79 (2.24, 3.48) | 2.16 (1.66, 2.80) | 3.13 (2.77, 3.54) | 2.40 (2.09, 2.75) |
| Antidiabetic agent adherence ≥80% §§,¶¶ | 2.79 (2.44, 3.19) | 2.54 (2.17, 2.98) | 2.65 (2.43, 2.89) | 2.18 (1.98, 2.41) |
| No hypertension | Reference | Reference | Reference | Reference |
| Hypertension diagnosed and nontreated §§ | 1.06 (0.77, 1.46) | 1.14 (0.80, 1.61) | 0.99 (0.79, 1.25) | 1.09 (0.86, 1.39) |
| New diagnosed hypertension§§ | 1.81 (1.52, 2.16) | 1.39 (1.14, 1.69) | 3.09 (2.56, 3.73) | 2.06 (1.68, 2.52) |
| Antihypertensive agent adherence <80%§§,¶¶ | 2.47 (2.01, 3.03) | 2.08 (1.64, 2.65) | 3.10 (2.72, 3.54) | 2.22 (1.92, 2.55) |
| Antihypertensive agent adherence ≥80%§§,¶¶ | 1.95 (1.70, 2.25) | 1.60 (1.36, 1.88) | 2.85 (2.55, 3.18) | 2.18 (1.94, 2.46) |
| Respiratory diseases (yes vs. no)§§ | 2.63 (2.31, 2.99) | 2.13 (1.83, 2.48) | 2.25 (2.08, 2.44) | 1.70 (1.55, 1.86) |
| Antidepressant drugs (yes vs. no)§§ | 1.45 (1.23, 1.71) | 1.19 (0.97, 1.45) | 1.40 (1.27, 1.55) | 1.07 (0.95, 1.20) |
| Anxiolytic drugs (yes vs. no)§§ | 1.39 (1.24, 1.56) | 1.13 (0.99, 1.31) | 1.42 (1.32, 1.53) | 1.14 (1.05, 1.25) |
| Chronic disease score (≥4 vs. <4) | 2.26 (1.94, 2.62) | 1.70 (1.42, 2.04) | 2.67 (2.46, 2.94) | 2.00 (1.81, 2.21) |
At treatment initiation.
Statins equivalent in simvastatin dose during follow-up simvastatin 20 mg = lovastatin 40 mg = pravastatin 40 mg = fluvastatin 80 mg = atorvastatin 10 mg = rosuvastatin 5 mg.
Proportion of days covered (%).
Diagnosis of cerebrovascular disease: (ICD-9 codes 430–438) or medical procedures.
Diagnosis of peripheral artery disease (PAD): diagnosis (ICD-9 codes 440–447), medical procedure of noncoronary angioplasty, or use of pentoxifylline).
Diagnosis of coronary artery disease (CAD): myocardial infarction or angina (ICD-9: 410–414), a medical procedure, i.e. coronary artery bypass grafting, angiography, or angioplasty, use of nitrate, including nitroglycerin.
Diagnosis of other cardiovascular disease (CVD): arrhythmia: diagnosis (ICD-9 code 427), a medical procedure using a pacemaker or the use of drugs for cardiac arrhythmias (amiodarone, digoxin, quinidine, disopyramide, flecainamide, mexiletine, procainamide, propafenone, or sotalol); or valvular heart disease; or anticoagulants.
ICD-9 or pharmacological treatment.
Proportion of days covered (%) in the year before the index date. The model was adjusted for all these variables.
Subgroup analysis revealed a similar effect among subjects <65 years old (RR 0.78; 0.62, 0.99) and those >65 years old (RR 0.81; 0.70, 0.94). The fact of being adherent for >80% decreased the risk of CHF among patients having been diagnosed with hypertension or diabetes (RR 0.80; 0.70, 0.90). Similar results were also observed among those without hypertension and diabetes (RR 0.60; 0.38, 0.95).
Sensitivity analyses
As shown in Table 4, we considered an unmeasured risk factor less frequent among subjects with a high adherence level compared with those with a low adherence level. In the scenarios with the highest probability (scenarios 5 and 6), with 15% smokers (8% current and 7% former smokers) in the high adherence level group compared with 25% smokers (18% current and 7% former smokers) in the low adherence level group and with risk factors ranging from 3.0 to 4.0 for current smokers and 2.0 for former smokers, we inverted the relationship to a nonsignificant adjusted RR. Only the highest probability of unmeasured confounders will invert the relationship to a nonsignificant RR; based upon those scenarios, bias of that magnitude is unlikely to be present.
Table 4.
Change in rate ratio (RR) of nonfatal chronic heart failure (CHF) event after adjustment for unmeasured confounders (Greenland's Monte-Carlo approach)
| Prevalence of risk factor* (high risk; medium risk)‡ | Odds ratio† (CI) | ||||
|---|---|---|---|---|---|
| Scenario | High adherence group | Low adherence group | High risk‡ | Medium risk‡ | Estimated RR of nonfatal CHF event |
| 1 | 15% (8%; 7%) | 19% (12%; 7%) | 1.8 (1.2, 2.3) | 1.3 (1.1, 1.8) | 0.84 (0.78, 0.91) |
| 2 | 15% (8%; 7%) | 19% (12%; 7%) | 2.5 (1.6, 3.5) | 2.0 (1.2, 3.0) | 0.85 (0.79, 0.92) |
| 3 | 15% (8%; 7%) | 25% (12%; 13%) | 1.8 (1.2, 2.3) | 1.3 (1.1, 1.8) | 0.85 (0.79, 0.91) |
| 4 | 15% (8%; 7%) | 25% (18%; 7%) | 1.8 (1.2, 2.3) | 1.3 (1.1, 1.8) | 0.87 (0.80, 0.94) |
| 5 | 15% (8%; 7%) | 25% (18%; 7%) | 3.0 (1.2, 4.0) | 2.0 (1.1, 3.0) | 0.94 (0.84, 1.08) |
| 6 | 15% (8%; 7%) | 25% (18%; 7%) | 4.0 (1.2, 5.0) | 2.0 (1.1, 3.0) | 1.00 (0.87, 1.18) |
Proportion at high risk and medium risk in high-adherence and low-adherence groups.
Risk factor between the confounder and CHF event.
High risk and medium risk are defined as smokers (proportion of current smokers or social smokers) or obesity (proportion of severe obesity or moderated).
Discussion
This is the first study to examine the association of the adherence to statins with the risk of developing CHF for primary prevention. The current results show that adherence to statins exceeding 80% is associated with a reduction of 19% in the rate of CHF. Other evidence supports the benefits of statins for CHF, but in secondary prevention. For example, a systematic review of observational designs has suggested that statins are associated with lower morbidity and mortality among patients with CHF [15]. First, a Canadian observational study in individuals who survived after hospitalization for CHF and who received at least one statin prescription within 90 days after discharge compared with nonusers was associated with a 28% lower adjusted risk of CVD composite outcome compared with non-users [46]. Second, the use of statin for 12 months was associated with a 59% relative risk reduction of death among patients with advanced ischaemic and non-ischaemic CHF [47]. Third, in a post hoc analysis of 1153 patients with severe systolic CHF of ischaemic and non-ischaemic aetiologies in the PRASE trial [48], statin use was associated with a 48% lower adjusted risk of death among 134 patients who had received statins for 1 year compared with non-users. Fourth, among adults with CHF, incident statin use was associated with a lower risk of death (24%) and hospitalization (21%) in secondary prevention of CVD among patients with or without CAD compared with non-users [49]. Fifth, compared with non-users, statin use was associated with a significant difference in the mortality rate in patients with heart failure and preserved left ventricular ejection fraction [50]. Sixth, in an elderly population with newly diagnosed congestive heart failure, statins presented similar effectiveness in preventing mortality [51]. Finally, statin therapy was also associated with a reduced risk of death in patients >40 years old who had undergone coronary angiography and with a physician-diagnosed history of heart failure [52].
Other evidence also supports the effect of statins on CHF. First, in the 4S Study trial, patients with CAD without CHF were randomized to simvastatin and placebo; in a post hoc analysis, the subsequent rates of CHF were 8.3 and 10.3%, respectively [53]. Second, in the Treating to New Targets study, atorvastatin at 80 mg compared with 10 mg in patients with stable coronary disease significantly reduced hospitalizations for heart failure defined as a secondary end-point [54]. Third, in a post hoc analysis of 5010 patients with CHF from the Val-HeFT study, statins appeared to be associated with a lower rate of mortality [55]. Finally, limited existing randomized comparisons in relatively small samples have yielded mixed results for intermediate markers related to inflammatory components, left ventricular function and other echographic parameters [16–19].
On the other hand, the recent CORONA study [56] has reported that among a population of older patients with moderate to severe ischaemic systolic heart failure, despite the favourable effects on LDL-cholesterol, high-density lipoprotein-cholesterol and triglyceride levels and on high-sensitivity C-reactive protein of 10 mg of rosuvastatin, no significant result was seen on the primary composite cardiovascular outcome or death from any cause, but a significant reduction in the number of hospitalizations for cardiovascular causes was observed.
The risk factors for CHF, such as the presence of hypertension, myocardial infarction or diabetes, agree with the findings from other studies [57, 58]. The coefficients associated with CHF risk factors such as hypertension, myocardial infarction and diabetes are 2.1 and 3.4, 6.3 and 6.0, and 1.4 and 3.7 for men for women, respectively. The risk of developing CHF in subjects with a predisposing medical condition (e.g. coronary disease, valve disease or hypertension) varies over a large range depending upon the related number of medical conditions of deteriorating cardiac function [59]. For example, the risk of CHF in individuals with hypertension varies over more than 10 times depending on the number of these associated risk factors. Again, a cluster of hypertension along with dyslipidaemia, diabetes and obesity yields a fourfold increase in the expected rate of CHF.
The current study has attempted to overcome some methodological problems. Given the concern about treatment for selection bias, we used only incident statin users. As with all observational studies on the effects of medications, the potential for confounding by indication should be carefully assessed. First, since the patients studied were all receiving statins, there is a lower likelihood of confounding by indication [60]. On the other hand, we could not control for all patient characteristics that could have influenced the choice of physician. Unmeasured comorbidity as well as missing clinical data related to cholesterol levels could confer residual confounding effects. However, there is no reason to believe that prescribing a different statin would be strongly influenced by the cholesterol level. The analysis of available baseline characteristics did not suggest preferential prescribing of a particular statin to sicker patients.
Second, patients with comorbidities may be more likely to be adherent to prescribed statin as well as being more likely to have CVD events. We controlled for CVD risk factors and the development of CVD events through the inclusion of relevant variables in the regression model to decrease the bias further. The covariables were the fact of having hypertensive status or being a diabetic patient, or of developing a CVD event after the initiation of statin agents. Our multivariate analysis should have minimized the influence of confounding factors. Nevertheless, residual confounding effect due to incomplete or inaccurate measurement of covariates or unmeasured confounders cannot be excluded. For example, patients who are non-adherent may have other traits that contribute to worsened outcomes, including factors such as depression, lower socioeconomic status and associated adverse health behaviours [61]. However, we were able to adjust, at least in part, for these factors.
The current study had other limitations. First, databases do not allow adjustment for clinical severity. We did not have any cholesterol values and thus could not adjust them before and after treatment. To investigate the possible bias, we evaluated the rate of switching to other doses and found that most patients (84%) were taking the same, whereas the rate of switching to other statins was 9%. Second, in order to reduce the likelihood of confounding by dose, we evaluated the equivalent simvastatin dose, and the doses were comparable among cases and controls. Furthermore, the observed profile of prescribing low doses limited our ability to compare statins at higher doses. Third, we could not adjust for blood pressure or glycaemia, well-known CHF risk factors. However, if patients were using drugs to treat hypertension or diabetes, we defined the categories of adherence levels for these therapies to take into account the adherence level and the risk reduction of CHF. Fourth, residual confounding by unmeasured factors is always possible. For example, the databases do not allow any adjustment for clinical severity such as left ventricular hypertrophy, ejection fraction and CHF aetiology. Fifth, RAMQ databases did not allow for lifestyle adjustments (e.g. smoking, lack of exercise). Since these factors are more likely to be present among patients who do not adhere to medications, they may introduce a bias [62]. The adherence level to medications may be a marker for a better prognosis [63–65]. Given that smoking data were not available for the regression modelling, we evaluated the robustness of our estimates. As shown (Table 4), only very large disparities in the smoking frequency in the two groups would be able to render the RR nonsignificant, and, based upon those scenarios, bias of that magnitude is unlikely to be present. Sixth, information bias may also be possible; for example, some subjects may have had a previous CVD that did not appear in the information on the 5-year period prior to the cohort entry. Therefore, it is reasonable to believe that our subjects may not have had a symptomatic CVD, giving that no drug markers were used in the 2 years before the cohort entry. Finally, a possible misclassification error may be related to statin exposure; patients pay a proportion of the drug costs, so they may be more likely to take their drug, lowering the chances of bias.
Despite those limitations, the study results provide an association of the impact of adherence to statins on CHF for primary prevention. It is important to raise the awareness of health professionals of the need to improve adherence to therapy. This study provides evidence of the potential role of statins in CHF.
Acknowledgments
The Canadian Institutes Health Research (CIHR) supported this work. S.P., L.L., L.B. and A.B. are research scholars who receive financial support from the Fonds de recherche en santé du Quéec.
REFERENCES
- 1.Fondation des maladies du Coeur du Canada (Fondation Des Maladies Du Coeur Du Canada) Le fardeau croissant des maladies cardiovasculaires et des accidents vasculaires céréraux au Canada, 2003. 2003. Report no. 1-89624-32-4.
- 2.Ho KKL, Anderson KM, Kannel WB, Grossman W, Levy D. Survival after the onset of congestive heart failure in Framingham Heart Study subjects. Circulation. 1993;88:107–15. doi: 10.1161/01.cir.88.1.107. [DOI] [PubMed] [Google Scholar]
- 3.Bourassa MG, Gurné O, Bangdiwala SI, Ghali JK, Young JB, Rousseau M, Johnstone DE, Yusuf S. Natural history and patterns of current practice in heart failure. J Am Coll Cardiol. 1992;22(Suppl. A):14A–19A. doi: 10.1016/0735-1097(93)90456-b. [DOI] [PubMed] [Google Scholar]
- 4.Gheorghiade M, Bonow RO. Chronic heart failure in the United States: a manifestation of coronary artery disease. Circulation. 1998;97:282–9. doi: 10.1161/01.cir.97.3.282. [DOI] [PubMed] [Google Scholar]
- 5.Fox KF, Cowie MR, Wood DA, Coats AJS, Gibbs JSR, Underwood SR, Turner RM, Poole-Wilson PA, Davies SW, Sutton GC. Coronary artery disease as the cause of incident heart failure in the population. Eur Heart J. 2001;22:228–36. doi: 10.1053/euhj.2000.2289. [DOI] [PubMed] [Google Scholar]
- 6.Pfeffer MA, Braunwald E. Ventricular remodelling after myocardial infarction: experimental observations and clinical implications. Circulation. 1990;81:1161–72. doi: 10.1161/01.cir.81.4.1161. [DOI] [PubMed] [Google Scholar]
- 7.Cholesterol Treatment Trialists. Efficacy of cholesterol lowering therapy in 18 686 people with diabetes in 14 randomised trials of statins: a meta-analysis. Lancet. 2008;371:117–25. doi: 10.1016/S0140-6736(08)60104-X. [DOI] [PubMed] [Google Scholar]
- 8.Bohm M, Hjalmarson A, Kjekshus J, Laufs U, McMurray J, van Veldhuisen DJ. Heart failure and statins – why do we need a clinical trial? Z Kardiol. 2005;94:223–30. doi: 10.1007/s00392-005-0210-9. [DOI] [PubMed] [Google Scholar]
- 9.Urbich C, Dimmeler S. Risk factors for coronary artery disease, circulating endothelial progenitor cells, and the role of HMG-CoA reductase inhibitors. Kidney Int. 2005;67:1672–6. doi: 10.1111/j.1523-1755.2005.00261.x. [DOI] [PubMed] [Google Scholar]
- 10.Strey CH, Young JM, Molyneux SL, George PM, Florkowski CM, Scott RS, Frampton CM. Endothelium-ameliorating effects of statin therapy and coenzyme Q10 reductions in chronic heart failure. Atherosclerosis. 2005;179:201–206. doi: 10.1016/j.atherosclerosis.2004.10.009. [DOI] [PubMed] [Google Scholar]
- 11.Tousoulis D, Antoniades C, Bosinakou E, Kotsopoulou M, Pitsavos C, Vlachopoulos C, Panagiotakos D, Stefanadis C. Effects of atorvastatin on reactive hyperemia and inflammatory process in patients with congestive heart failure. Atherosclerosis. 2005;178:359–63. doi: 10.1016/j.atherosclerosis.2004.08.037. [DOI] [PubMed] [Google Scholar]
- 12.Rundek T, Naina A, Sacco R, Coates K, DiMauro S. Atorvastatin decreases the coenzyme Q10 level in the blood of patients at risk for cardiovascular disease and stroke. Arch Neurol. 2004;61:889–92. doi: 10.1001/archneur.61.6.889. [DOI] [PubMed] [Google Scholar]
- 13.Moosmann B, Behl C. Selenoprotein synthesis and side-effects of statins. Lancet. 2004;363:892–4. doi: 10.1016/S0140-6736(04)15739-5. [DOI] [PubMed] [Google Scholar]
- 14.Raunchhaus M, Coats AJ, Anker SD. The endotoxin-lipoprotein hypothesis. Lancet. 2000;356:930–3. doi: 10.1016/S0140-6736(00)02690-8. [DOI] [PubMed] [Google Scholar]
- 15.Van der Harst P, Voors AA, van Gilst WH, Bohm M, van Veldhuisen DJ. Statins in the treatment of chronic heart failure: a systematic review. PLoS Med. 2006;3:e333. doi: 10.1371/journal.pmed.0030333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bleske BE, Nicklas JM, Bard RL, Brook RD, Gurbel PA, Bliden KP, Rajagopalan S, Pitt B. Neutral effect on markers of heart failure, inflammation, endothelial activation and function, and vagal tone after high dose HMG-CoA reductase inhibition in non diabetic patients with non-ischemic cardiomyopathy average low-density lipoprotein level. J Am Coll Cardiol. 2006;47:338–41. doi: 10.1016/j.jacc.2005.06.087. [DOI] [PubMed] [Google Scholar]
- 17.Mozaffarian D, Minami E, Letterer RA, Lawler RL, McDonald GB, Levy WC. The effects of atorvastatin (10 mg) on systemic inflammation in heart failure. Am J Cardiol. 2005;96:1699–704. doi: 10.1016/j.amjcard.2005.07.092. [DOI] [PubMed] [Google Scholar]
- 18.Sola S, Mir MQ, Lerakis S, Tandon N, Khan BV. Atorvastatin improves left ventricular systolic function and serum markers of inflammation in nonischemic heart failure. J Am Coll Cardiol. 2006;47:332–7. doi: 10.1016/j.jacc.2005.06.088. [DOI] [PubMed] [Google Scholar]
- 19.Vrtovec B, Okrajsek R, Golicnik A, Ferjan M, Starc V, Radovancevic B. Atorvastatin therapy increases heart rate variability, decreases QT variability, and shortens QTC interval duration in patients with advanced chronic heart failure. J Am Coll Cardiol. 2005;11:684–90. doi: 10.1016/j.cardfail.2005.06.439. [DOI] [PubMed] [Google Scholar]
- 20.International Classification of Diseases. Manual of the International Statistical Classification of Diseases, Injuries, and Cause of Death. 9th edn. Geneva, Switzerland: World Health Organization; 1977. (Publication no PHS 80-1260. [Google Scholar]
- 21.Canada: Supply and Services. Canadian classification of diagnostic, therapeutic, and surgical procedures. 2nd edn. Ottawa, Ontario, Canada: Statistics Canada Health Division; 1986. [Google Scholar]
- 22.Régie de l'assurance maladie. Liste des médicaments. 15th edn. Québec, Canada: Régie de l'assurance maladie; 2003. [Google Scholar]
- 23.Régie de l'assurance maladie du Québec. Rapport annuel de gestion 2003–2004. Québec, Canada: Government of Quebec; 2004. [Google Scholar]
- 24.Tamblyn R, Lavoie G, Petrella L, Monette J. The use of prescription claims databases in pharmacoepidemiological research: the accuracy and comprehensiveness of the prescription claims database in Quebec. J Clin Epidemiol. 1995;48:999–1009. doi: 10.1016/0895-4356(94)00234-h. [DOI] [PubMed] [Google Scholar]
- 25.Wilchesky M, Tamblyn RM, Huang A. Validation of diagnostic codes within medical services claims. J Clin Epidemiol. 2004;57:131–41. doi: 10.1016/S0895-4356(03)00246-4. [DOI] [PubMed] [Google Scholar]
- 26.Tamblyn R, Reid T, Mayo N, McLeod P, Churchill-Smith M. Using medical services claims to assess injuries in the elderly: sensitivity of diagnostic and procedure codes for injury ascertainment. J Clin Epidemiol. 2000;53:183–94. doi: 10.1016/s0895-4356(99)00136-5. [DOI] [PubMed] [Google Scholar]
- 27.Monfared A, Rahme E, LeLorier J. Accuracy of ICD-9 diagnosis code ‘410’ to identify episodes of hospitalizations for acute myocardial infarction in RAMQ. First Canadian Therapeutics Congress 2004, Halifax, Canada, 2004.
- 28.Lee WY, Capra AM, Jensvold NG, Gurwitz JH. Go AS for the Epidemiology, Practice, Outcomes, and Costs of Heart Failure (EPOCH) Study. Am J Cardiol. 2004;94:1147–52. doi: 10.1016/j.amjcard.2004.07.081. [DOI] [PubMed] [Google Scholar]
- 29.McKee PA, Castelli WP, McNamara PM, Kannel WB. The natural history of congestive heart failure: the Framingham study. N Engl J Med. 1971;285:1441–6. doi: 10.1056/NEJM197112232852601. [DOI] [PubMed] [Google Scholar]
- 30.Lubin J, Gail M. Biased selection of controls for case control analyses of cohort studies. Biometrics. 1984;40:63–75. [PubMed] [Google Scholar]
- 31.Peterson AM, Nau DP, Cramer JA, Peterson AM, Nau DP, Cramer JA, Benner J, Gwadry-Sridhar F, Nichol M. A checklist for medication compliance and persistence studies using retrospective databases. Value Health. 2007;10:3–12. doi: 10.1111/j.1524-4733.2006.00139.x. [DOI] [PubMed] [Google Scholar]
- 32.Sikka R, Xia F, Aubert RE. Estimating medication persistency using administrative claims data. Am J Manag Care. 2005;11:449–57. [PubMed] [Google Scholar]
- 33.Huybrechts KF, Ishak KJ, Caro JJ. Assessment of compliance with osteoporosis treatment and its consequences in a managed care population. Bone. 2006;38:922–8. doi: 10.1016/j.bone.2005.10.022. [DOI] [PubMed] [Google Scholar]
- 34.LaRosa JC, He J, Vupputuri S. Effect of statins risk of coronary disease: a meta-analysis of randomized controlled trials. JAMA. 1999;282:2340–6. doi: 10.1001/jama.282.24.2340. [DOI] [PubMed] [Google Scholar]
- 35.Kendrach M, Kelly-Freeman M. Approximate equivalent rosuvastatin doses for temporary statin interchange programs. Ann Pharmacother. 2004;38:1286–92. doi: 10.1345/aph.1D391. [DOI] [PubMed] [Google Scholar]
- 36.Jones PH, Davidson MH, Stein EA, Bays HE, McKenney JM, Miller E, Cain VA, Blasetto JW. Comparison of the efficacy and safety of rosuvastatin versus atorvastatin, simvastatin, and pravastatin across doses (STELLAR Trial) Am J Cardiol. 2003;92:152–60. doi: 10.1016/s0002-9149(03)00530-7. [DOI] [PubMed] [Google Scholar]
- 37.Von Korff M, Wagner E, Saunders K. A chronic disease score from automated pharmacy data. J Clin Epidemiol. 1992;45:197–203. doi: 10.1016/0895-4356(92)90016-g. [DOI] [PubMed] [Google Scholar]
- 38.Essebag V, Platt R, Abrahamowicz M, Pilote L. Comparison of nested case–control and survival analysis methodologies for analysis of time-dependent exposure. BMC Med Res Method. 2005;5:5–11. doi: 10.1186/1471-2288-5-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Greenland S. Sensitivity analysis, Monte Carlo risk analysis and Bayesian uncertainty assessment. Risk Anal. 2001;21:579–83. doi: 10.1111/0272-4332.214136. [DOI] [PubMed] [Google Scholar]
- 40.Steenland K, Greenland S. Monte Carlo Sensitivity Analysis and Bayesian Analysis of Smoking as an unmeasured confounder in a study of Silica and Lung Cancer. Am J Epidemiol. 2004;160:384–92. doi: 10.1093/aje/kwh211. [DOI] [PubMed] [Google Scholar]
- 41.Kiortsis DN, Bruckert GE, Turpin G. Factors associated with low compliance with lipid-lowering drugs in hyperlipidemic patients. J Clin Pharm Ther. 2000;25:445–51. doi: 10.1046/j.1365-2710.2000.00315.x. [DOI] [PubMed] [Google Scholar]
- 42.Kirkland SA, Maclean DR, Langille DB, Joffres MR, McPherson KM, Andreou P. Knowledge and awareness of risk factors for cardiovascular disease among Canadians 55–74 years of age: results from the Canadian heart Health Surveys, 1986–1992. CMAJ. 1999;161:S10–6. [PMC free article] [PubMed] [Google Scholar]
- 43.Penning-van Beest FJA, Termorshuizen F, Goettsch WG, Klungel OH, Kastelein JJP, Herings RMC. Adherence to evidence-based statin guidelines reduces the risk of hospitalizations for acute myocardial infarction by 40%: a cohort study. Eur Heart J. 2007;28:154–9. doi: 10.1093/eurheartj/ehl391. [DOI] [PubMed] [Google Scholar]
- 44.Greene WH. Econometric Analysis. 3rd edn. Upper Saddle River, NJ: Prentice Hall; 1997. p. 552. [Google Scholar]
- 45.Belsley DA, Kuy E, Welsch RE. Regression Diagnostics: Identifying Influential Data and Sources of Collinearity. New-York: John Wiley & Sons; 1981. [Google Scholar]
- 46.Ray JG, Gong Y, Sykora K, Tu JV. Statin use and survival outcomes in elderly patients with heart failure. Arch Intern Med. 2005;165:62–7. doi: 10.1001/archinte.165.1.62. [DOI] [PubMed] [Google Scholar]
- 47.Horwich TB, MacLellan WR, Fonarow GC. Statin therapy is associated with improved survival in ischemic and non-ischemic heart failure. J Am Coll Cardiol. 2004;43:642–8. doi: 10.1016/j.jacc.2003.07.049. [DOI] [PubMed] [Google Scholar]
- 48.Mozaffarian D, Nye R, Levy WC. Statin therapy is associated with lower mortality among patients with severe heart failure. Am J Cardiol. 2004;93:1124–9. doi: 10.1016/j.amjcard.2004.01.039. [DOI] [PubMed] [Google Scholar]
- 49.Go AS, Lee WY, Yang J, Lo JC, Gurwitz JH. Statin therapy and risks for death and hospitalization in chronic heart disease. JAMA. 2006;296:2105–17. doi: 10.1001/jama.296.17.2105. [DOI] [PubMed] [Google Scholar]
- 50.Shah R, Wang Y, Foody JM. Effect of statins, angiotensin-converting enzyme inhibitors, and beta blockers on survival in patients ≥65 years of age with heart failure and preserved left ventricular systolic function. Am J Cardiol. 2008;101:217–22. doi: 10.1016/j.amjcard.2007.08.050. [DOI] [PubMed] [Google Scholar]
- 51.Rinfret S, Behlouli H, Eisenberg MJ, Humphries K, Tu JV, Pilote L. Class effects of statins in elderly patients with congestive heart failure: a population based analysis. Am Heart J. 2008;155:316–23. doi: 10.1016/j.ahj.2007.09.004. [DOI] [PubMed] [Google Scholar]
- 52.Ray JG, Norris CM, Udell JA, Tsuyuki RT, McAlister FA, Knudtson ML, Ghali WA. Lipid lowering therapy and outcomes in heart failure. J Cardiolvasc Pharmacol Ther. 2007;12:27–35. doi: 10.1177/1074248407299839. [DOI] [PubMed] [Google Scholar]
- 53.Scandanavian Simvastatin Survival Study Group. Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S) Lancet. 1994;344:1383–9. [PubMed] [Google Scholar]
- 54.Khush KK, Waters DD, Bittner V, Deedwania PC, Kastelein JJP, Lewis SJ, Wenger NK. Effect of high dose atorvastatin on hospitalizations for heart failure: subgroup analysis of the Treating to New Targets (TNT) study. Circulation. 2007;115:576–83. doi: 10.1161/CIRCULATIONAHA.106.625574. [DOI] [PubMed] [Google Scholar]
- 55.Krum H, Latini R, Maggioni AP, Anand I, Masson S, Carretta E, Ingrilli F, Pettinati G, Glazer R, Tognoni G, Cohn J. Statins and symptomatic chronic systolic heart failure: a post hoc analysis of 5010 patients enrolled in Val-HeFT. Int J Cardiol. 2007;119:48–53. doi: 10.1016/j.ijcard.2006.07.106. [DOI] [PubMed] [Google Scholar]
- 56.Kjekshus J, Apetrei E, Barrios V, Böhm M, Cleland JG, Cornel JH, Dunselman G, Fonseca C, Goudev A, Grande P, Gullestad L, Hjalmarson A, Hradec A, Jánosi A, Kamensky G, Komajda M, Korewicki J, Kuusi T, Mach F, Mareev V, McMurray JJ, Ranjith N, Schaufelberger M, Vanhaecke J, Van Veldhuisen DJ, Waagstein F, Wedel H, Wikstrand J. Rosuvastatin in older patients with systolic heart failure. N Engl J Med. 2007;357:2248–61. doi: 10.1056/NEJMoa0706201. [DOI] [PubMed] [Google Scholar]
- 57.Kannel WB. Incidence and epidemiology of heart failure. Heart Failure Rev. 2000;5:167–73. doi: 10.1023/A:1009884820941. [DOI] [PubMed] [Google Scholar]
- 58.Ho KK, Pinsky JL, Kannel WB, Levy D. The epidemiology of heart failure: the Framingham Heart Study. J Am Coll Cardiol. 1993;22:6A–13A. doi: 10.1016/0735-1097(93)90455-a. [DOI] [PubMed] [Google Scholar]
- 59.Kannel WB, Béanger AJ. Epidemiology of heart failure. Am Heart J. 1991;121:951–7. doi: 10.1016/0002-8703(91)90225-7. [DOI] [PubMed] [Google Scholar]
- 60.Salas M, Hofman A, Stricker BH. Confounding by indication: an example of variation in the use of epidemiology terminology. Am J Epidemiol. 1999;149:981–3. doi: 10.1093/oxfordjournals.aje.a009758. [DOI] [PubMed] [Google Scholar]
- 61.Osterberg L, Blaschke T. Adherence to medication. N Engl J Med. 2005;353:487–97. doi: 10.1056/NEJMra050100. [DOI] [PubMed] [Google Scholar]
- 62.Kim YS, Sunwoo S, Lee HR, Park YW, Shin HC, Kim CH, Kim DH, Kim BS, Cha HS, Huh BY. Determinants of non-compliance with lipid-lowering therapy in hyperlipidemic patients. Pharmacoepidemiol Drug Saf. 2002;11:593–600. doi: 10.1002/pds.730. [DOI] [PubMed] [Google Scholar]
- 63.McDermott MM, Schmitt B, Wallner E. Impact of medication nonadherence on coronary heart disease outcomes. A critical review. Arch Intern Med. 1997;157:1921–9. [PubMed] [Google Scholar]
- 64.Influence of adherence to treatment and response of cholesterol on mortality in the coronary drug project. N Engl J Med. 1980;303:1038–41. doi: 10.1056/NEJM198010303031804. [DOI] [PubMed] [Google Scholar]
- 65.Middleton A, Fuat A. Achieving lipid goals in real life: the DISCOVERY-UK Study. Br J Cardiol. 2006;13:72–6. [Google Scholar]


