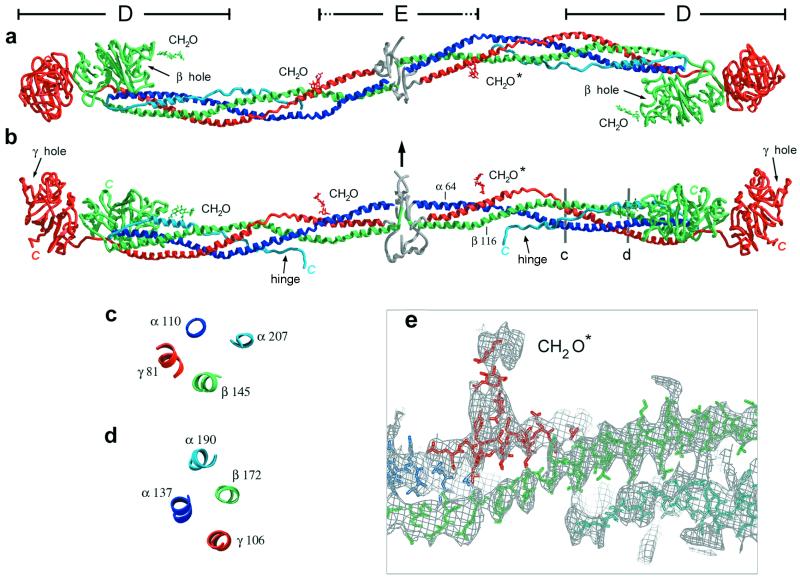
Figure 1.
Three perpendicular views of the modified bovine fibrinogen molecule. Color scheme (for all figures) is blue for the Aα-chain, green for the Bβ-chain, and red for the γ-chain. The antiparallel portion of the Aα-chain is shown in light blue. The chains in the central N-terminal disulfide knot region cannot be traced and their general location is indicated in gray. Carbohydrates are indicated by CH2O. (Bovine residue numbers are indicated, see Methods.) (a) View showing the sigmoidal shape of the coiled-coil axis. (b) View perpendicular to that in a indicating the molecular planarity. Arrow indicates approximate 2-fold axis of the molecule, which is nearly perpendicular to the plane of the sigmoidal coiled coil. Observed C termini are indicated in italics. (c and d) Cross-sectional views of the four-stranded coiled coil at locations indicated in b. The four-stranded coiled coil is quite asymmetric and may be better described as an overlapping pair of three-stranded coiled coils that share the parallel Aα- and Bβ-chains. (The γ and antiparallel Aα-chains do not contact one another.) (e) Magnified view of b showing part of the coiled coil superimposed on an electron density map (see Methods). A polyalanine model in this segment of the antiparallel Aα-chain density (Bottom, light blue) is shown. Regular helical density is observed for the parallel chains.