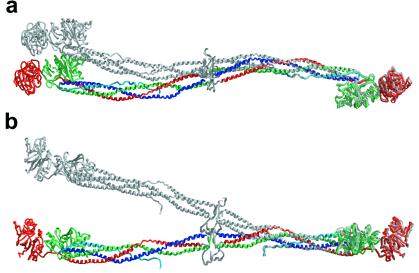
Figure 2.
Conformational flexibility of fibrinogen in the crystals. The diagrams show superpositions of noncrystallographically related fibrinogen molecules based on the least-squares fit of the relatively rigid coiled-coil segment: Aα104-Aα154, Bβ140-Bβ190, γ77-γ127. Among the different noncrystallographically related copies, the rms difference between the coordinates of this segment is about half that of the backbone's most flexible segment: Aα64-Aα114, Bβ100-Bβ150, γ37-γ87. (a) View, as in Fig. 1a, of one pair of molecules whose conformations differ primarily by bending within the plane of the sigmoidal coiled-coil axis. (b) View, as in Fig. 1b, of a different pair of molecules whose conformations differ primarily by bending out of the plane of the sigmoidal axis.