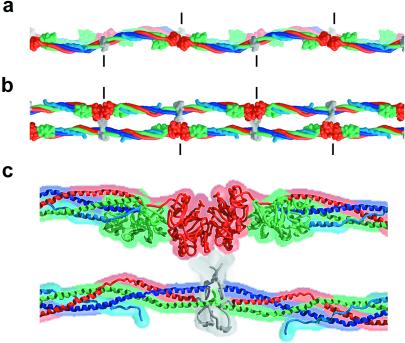
Figure 4.
A model of the two-stranded protofibril of fibrin derived from a filament in the bovine fibrinogen crystal structure. (a) View as in Fig. 1a. The sigmoidal coiled-coil axes of the two filaments are in phase. (b) View as in Fig. 1b. (c) Magnified view of b, including the DDE cluster. The model was constructed so that the two filaments are half-staggered (by 225 Å), closed (the γ-domain receptor pockets face the adjacent filament), and separated by ≈60 Å (a rough estimate from figure 9 in ref. 4 and J. Weisel, personal communication). The contact area between the D and E regions is not known and is illustrated schematically here. Short vertical lines indicate the molecular boundaries. The ≈30-Å distance between the N terminus of the Aα-chain coiled coil from one filament and the γ-domain hole on the other filament (into which the GPR sequence binds) could easily be spanned by the 30 additional residues in the disulfide knot region. Similarly, the ≈100-Å distance between the N terminus of the Bβ-chain coiled coil and the β-domain receptor pocket of the adjacent filament also could be bridged by the 64 residues in this region. This long distance between the disulfide knot and the GHR sequence of the Bβ-chain also may allow binding between protofibrils.