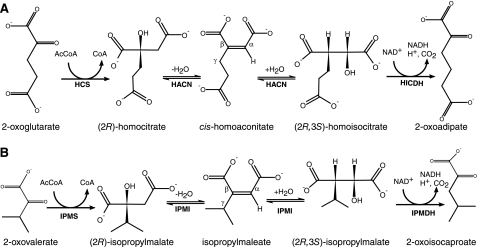
FIGURE 1.
Biosynthetic 2-oxoacid elongation pathways. A, three enzymes convert 2-oxoglutarate to 2-oxoadipate in the α-aminoadipate pathway and the biosynthesis of the CoB acyl chain. HCS (MJ0503) catalyzes the aldol-type addition of an acetyl group to the re-face of 2-oxoglutarate, producing (R)-homocitrate. Aconitase or methanogen HACN (MJ1003 and MJ1271) catalyzes the anti-elimination of the hydroxyl group and pro-R hydrogen from homocitrate forming the intermediate cis-homoaconitate. All of the HACN enzymes catalyze the anti-addition of water to produce (2R,3S)-homoisocitrate. Finally, HICDH (MJ1596) catalyzes an oxidative decarboxylation reaction forming 2-oxoadipate. Replacing 2-oxoglutarate with 2-oxoadipate produces 2-oxopimelate, and a third iteration of this pathway produces 2-oxosuberate. B, the analogous reactions in leucine biosynthesis in M. jannaschii use isopropylmalate synthase (IPMS; MJ1195), IPMI (MJ0499 and MJ1277), and isopropylmalate dehydrogenase (IPMDH; MJ0720). The citramalate synthase (MJ1392) replaces isopropylmalate synthase in isoleucine biosynthesis, using pyruvate to produce (R)-citramalate that is converted to 2-oxobutyrate by IPMI and isopropylmalate dehydrogenase (28). The conserved α and β groups and the variable γ groups of cis-homoaconitate and isopropylmaleate are labeled for reference.