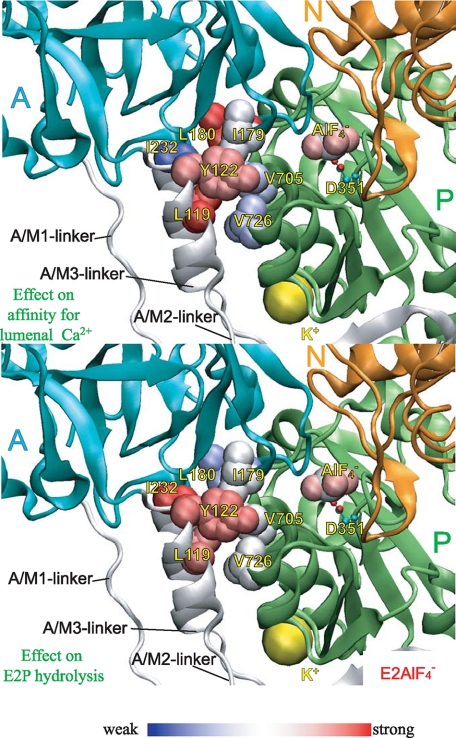
FIGURE 10.
Strength of the mutational effects of seven residues in
Tyr122-hydrophobic cluster on E2P hydrolysis and lumenal
Ca2+ affinity. The detailed structure at Y122-HC is shown with
 (the analog for
E2∼P, the transition state of the E2P hydrolysis
(21), PDB code: 1XP5
(15)). The seven residues
involved in Y122-HC (Tyr122/Leu119,
Ile179/Leu180, Ile232, and
Val705/Val726),
(the analog for
E2∼P, the transition state of the E2P hydrolysis
(21), PDB code: 1XP5
(15)). The seven residues
involved in Y122-HC (Tyr122/Leu119,
Ile179/Leu180, Ile232, and
Val705/Val726),
 bound at the phosphorylation site
Asp351, and the bound potassium ion are shown by van der Waals
spheres. The seven residues in Y122-HC are colored
differently based on the strength of the retardation of the E2P
hydrolysis rate (lower panel) and that of the increase in the lumenal
Ca2+ affinity (upper panel). The color changes
gradually from red for the strongest effects to blue for
weakening.
bound at the phosphorylation site
Asp351, and the bound potassium ion are shown by van der Waals
spheres. The seven residues in Y122-HC are colored
differently based on the strength of the retardation of the E2P
hydrolysis rate (lower panel) and that of the increase in the lumenal
Ca2+ affinity (upper panel). The color changes
gradually from red for the strongest effects to blue for
weakening.