FIGURE 1.
Structure of SERCA1a and formation of Tyr122-hydrophobic
cluster. The coordinates for the structures
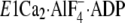 (the analog for the transition state of the phosphoryl transfer
E1∼PCa2·ADP, left panel) and
(the analog for the transition state of the phosphoryl transfer
E1∼PCa2·ADP, left panel) and
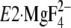 (E2·Pi analog
(21), right panel) of
Ca2+-ATPase were obtained from the Protein Data Bank (PDB accession
codes 1T5T and 1WPG, respectively
(12,
14)). The arrows
indicate approximate movements of the A and P domain and the top part of M2
(Leu119/Tyr122) in the change from
(E2·Pi analog
(21), right panel) of
Ca2+-ATPase were obtained from the Protein Data Bank (PDB accession
codes 1T5T and 1WPG, respectively
(12,
14)). The arrows
indicate approximate movements of the A and P domain and the top part of M2
(Leu119/Tyr122) in the change from
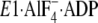 to
to
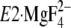 . The seven hydrophobic
residues, Ile179/Leu180/Ile232 on the A
domain, Leu119/Tyr122 on the A/M2-linker,
Val705/Val726 on the P domain are depicted as van der
Waals spheres. They gather to form a hydrophobic cluster around
Tyr122 in the change
. The seven hydrophobic
residues, Ile179/Leu180/Ile232 on the A
domain, Leu119/Tyr122 on the A/M2-linker,
Val705/Val726 on the P domain are depicted as van der
Waals spheres. They gather to form a hydrophobic cluster around
Tyr122 in the change
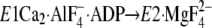 (Y122-HC, surrounded by the red dotted circle). The top part of M2,
including Leu119/Tyr122, is unwound in
(Y122-HC, surrounded by the red dotted circle). The top part of M2,
including Leu119/Tyr122, is unwound in
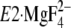 ,
,
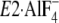 , and
, and
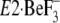 with bound thapsigargin
(TG), and thus becomes the A/M2-linker loop (see the region of M2 colored by
pink).
with bound thapsigargin
(TG), and thus becomes the A/M2-linker loop (see the region of M2 colored by
pink).

