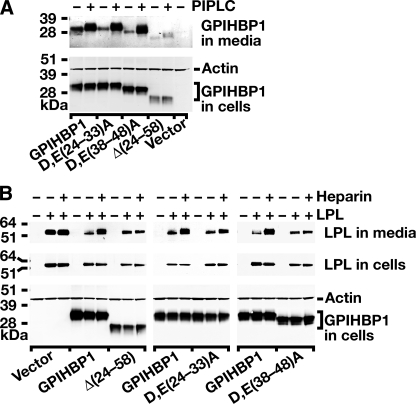
FIGURE 4.
Mutations in the acidic domain of GPIHBP1 prevent LPL binding. A, an S-protein-tagged wild-type GPIHBP1 construct or mutant GPIHBP1 constructs ((D,E(24-33)A, D,E(38-48)A, Δ(24-58)) were transiently transfected into CHO pgsA-745 cells, and Western blots were performed on cell extracts and the cell culture medium with an antibody against the S-protein tag. To determine whether GPIHBP1 reached the surface of the cell, cells were incubated with PIPLC (1 units/ml for 1 h at 37°C), and the appearance of GPIHBP1 in the medium was assessed by Western blot. PIPLC released similar amounts of wild-type GPIHBP1, D,E(24-33)A GPIHBP1, and D,E(38-48)A GPIHBP1 into the medium. Δ(24-58) GPIHBP1 was expressed at lower levels in cells, and PIPLC released smaller amounts of that protein into the medium. B, Western blot analysis of CHO pgsA-745 cells that had been transfected with a V5-tagged human LPL expression vector, along with wild-type or mutant GPIHBP1 constructs. 24 h after the transfection, cell extracts and culture media were harvested and analyzed by Western blotting. After quantifying the bands on an Odyssey infrared scanner (Li-Cor), we determined that heparin treatment led to an 11.75-fold increase in the amount of LPL in the culture medium of cells expressing wild-type GPIHBP1. In the case of Δ(24-58) GPIHBP1 or D,E(38-48)A GPIHBP1, heparin treatment led to negligible changes in the amount of LPL released into the medium (0.97 and 1.1 of the amount at baseline, respectively). In cells expressing D,E(24-33)A GPIHBP1, LPL levels in the medium after heparin increased roughly 2-fold.