Abstract
The voltage-gated potassium channel Kv1.3 constitutes an attractive pharmacological target for the treatment of effector memory T cell-mediated autoimmune diseases such as multiple sclerosis and psoriasis. Using 5-methoxypsoralen (5-MOP, 1), a compound isolated from Ruta graveolens, as a template we previously synthesized 5-(4-phenoxybutoxy)psoralen (PAP-1, 2) which inhibits Kv1.3 with an IC50 of 2 nM. Since PAP-1 is more than 1000-fold more potent than 5-MOP, we here investigated whether attaching a 4-phenoxybutoxy side-chain to other heterocyclic systems would also produce potent Kv1.3 blockers. While 4-phenoxybutoxy substituted quinolines, quinazolines and phenanthrenes were inactive, 4-phenoxybutoxy substituted quinolinones, furoquinolines, coumarins or furochromones inhibited Kv1.3 with IC50s of 150 nM to 10 µM in whole-cell patch-clamp experiments. Our most potent new compound is 4-(4-phenoxybutoxy)-7H-furo[3,2-g]chromene-7-thione (73, IC50 17 nM), in which the carbonyl oxygen of PAP-1 is replaced by sulfur. Taken together, our results demonstrate that the psoralen system is a crucial part of the pharmacophore of phenoxyalkoxypsoralen-type Kv1.3 blockers.
Keywords: Kv1.3, voltage-gated potassium channel, PAP-1, immunosuppression
1. Introduction
The voltage-gated Kv1.3 and the calcium-activated KCa3.1 channel play an important role in T cell activation by providing the counter-balancing potassium efflux for the calcium influx which is necessary for T cell activation [1]. However, which of these two potassium channels is the dominating player in these events depends on the activation and the differentiation state of the respective T cell. While CCR7+ naive and central memory T cells (TCM), which mediate acute immune responses, up-regulate KCa3.1 following activation, CCR7− effector memory T cells (TEM) up-regulate Kv1.3 and rely on this channel for their calcium signaling and proliferation [2,3]. Kv1.3 blockade has therefore been proposed as a promising pharmacological approach for the selective suppression of TEM cell activation in T cell-mediated autoimmune diseases such as type-1 diabetes, rheumatoid arthritis and multiple sclerosis, where TEM cells are involved in the pathogenesis [3,4]. In proof of this therapeutic concept Kv1.3 blocking peptides such as the scorpion toxins kaliotoxin and margatoxin and the sea anemone toxin ShK and its more Kv1.3 selective derivative ShK(L5) have been demonstrated to effectively prevent or treat delayed-type hypersensitivity [5,6], experimental autoimmune encephalomyelitis [6–8], pristane-induced arthritis [3] and experimental periodontal bone resorption [9] in rats. Although, many of these peptides exhibit exquisite potency and selectivity and could potentially be developed into drugs given the advances in peptide delivery techniques, several pharmaceutical companies and academic groups have attempted to identify non-peptidic small molecule Kv1.3 blockers. The best known of the “industrial” Kv1.3 blockers are Pfizer’s CP-339818 and UK-78282 and Merck’s nor-triterpene correolide and cyclohexyl-substituted benzamides (PACs) (see a recent book chapter [10] and several reviews [1,11]). However, none of these compounds are potent and selective enough for development into drugs.
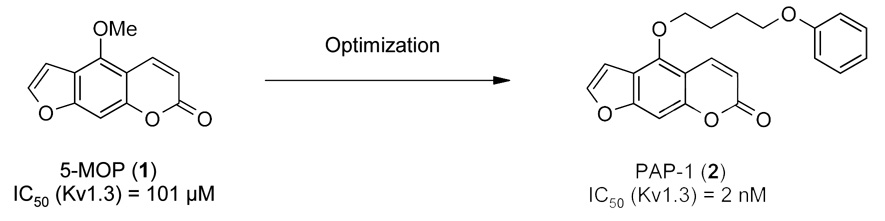
In the late 1980s anecdotal reports appeared from Chile and Austria, that drinking tea prepared from the leaves of Ruta graveolens, the common rue or the Herb of Grace, supposedly alleviated the symptoms of multiple sclerosis. During the next few years the groups of Wolfram Hänsel and Eilhard Koppenhöfer screened extracts from Ruta and eventually identified various psoralens as the potassium channel blocking principle of the plant [12,13]. The main ingredient, 5-methoxypsoralen (5-MOP, 1), a drug that is used in Europe for the treatment of psoriasis, was shown to block Kv1.3 and other Kv1-family channels in the micromolar range [14] and to reduce visual field defects in single-case studies in multiple sclerosis patients [15]. Since 5-MOP’s phototoxicity precluded its use as a drug for multiple sclerosis, we synthesized a large number of differently substituted psoralens with the aim of eliminating photoreactivity and increasing potency and selectivity for Kv1.3 [14,16,17]. As a result of this effort an over 1000-fold increase in potency in comparison to 5-MOP (1) was achieved by exchanging the methoxy group in 5-position with a 4-phenoxybutoxy group (see Figure 1). This 5-(4-phenoxybutoxy)psoralen (PAP-1, 2) blocks Kv1.3 with an IC50 of 2 nM, is 23-fold selective over the cardiac Kv1.5 channel (IC50 45 nM), 33- to 125-fold selective over Kv1.1, Kv1.2, Kv1.4, Kv1.6 and Kv1.7 and more than 1000-fold selective over more distantly related channels like HERG (Kv11.1), Na+, Ca2+ and Cl− channels [17]. PAP-1 further does not display any in vitro or in vivo toxicity [17], is orally available and has a half-life of 3 hours in rats and of 6.7 hours in rhesus macaques [3,18]. In keeping with its inhibitory effect on TEM cell function in vitro [3,17], PAP-1 also effectively suppresses oxazolone-induced allergic contact dermatitis [19] and significantly delays the onset and reduces the incidence of type-1 diabetes [3] in rat models. Based on these results our laboratory has proposed PAP-1 as a potential new drug for the topical treatment of psoriasis and potentially for the oral treatment of other TEM cell mediated autoimmune diseases [19,20]. However, despite the fact that we demonstrated that PAP-1 is not phototoxic [17] and that administration for 28 days or 6 months does not lead to any changes in liver enzymes or liver pathology in rats or rhesus macaques [3,18], pharmaceutical companies have been very hesitant to develop PAP-1 because psoralens are generally regarded as “toxicophores” due to the possibility that the coumarin ring could act as a Michael acceptor in reactions with glutathione and thus could potentially cause liver toxicity. There further is the possibility that the psoralen system could be epoxidated by cytochrome P450 enzymes and thus generate reactive metabolites.
Figure 1.
Design of PAP-1 based on the template 5-MOP.
We are therefore here searching for alternative heterocyclic systems to replace the psoralen system of PAP-1. Since attachment of the 4-phenoxybutoxy side-chain in 5-position of 5-MOP led to the above described dramatic increase in potency, we specifically investigated whether attaching a 4-phenoxybutoxy side-chain to other aromatic and/or heterocyclic scaffolds, different from the psoralen structure, would also result in potent Kv1.3 blockers. In order to determine that we were not loosing selectivity for Kv1.3 over the cardiac Kv1.5 channel, which exhibits a very high degree of homology to Kv1.3 in the pore region of the channel [1,10,11], we counter-screened all newly synthesized compounds on Kv1.5.
2. Chemistry
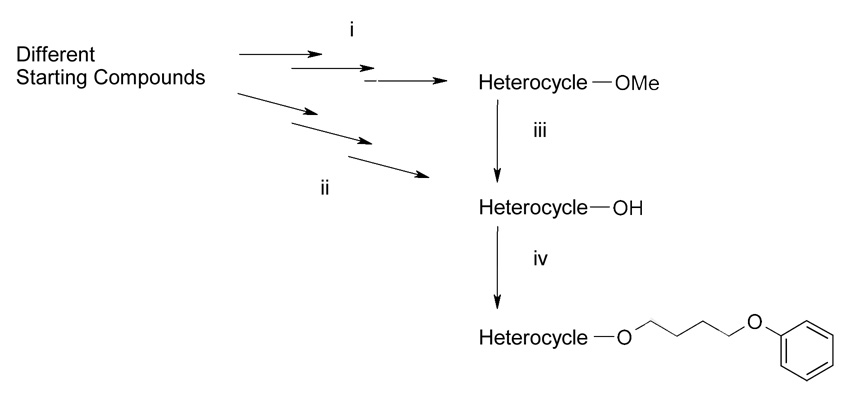
We here synthesized a series of 4-phenoxybutoxy substituted aromatic and heteroaromatic systems. For this purpose mono-, bi- and tricyclic ring systems containing either just carbon atoms or the heteroatoms nitrogen, oxygen and sulfur were used as scaffolds. A general overview of the synthesis sequences is given in scheme 1.
Scheme 1.
General Strategy of Synthesis.
Reagents: (i) scheme 6–scheme 8, (ii) scheme 5, (iii) scheme 4, (iv) scheme 2 and scheme 3.
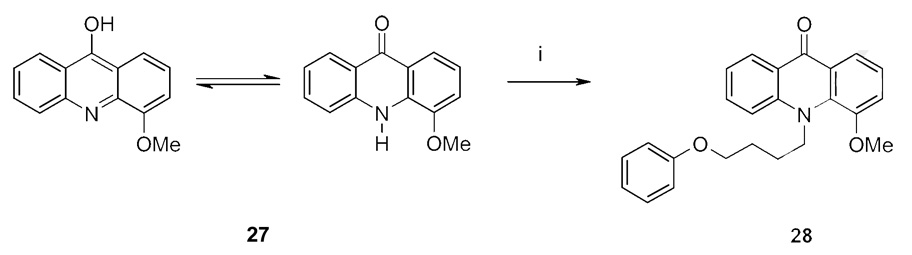
In cases where the desired scaffolds were commercially available as aliphatic or phenolic alcohols, the 4-phenoxybutoxy side-chain could be directly attached in a classic Williamson ether synthesis or in an analogous reaction using potassium carbonate or CsF-celite as solid base (Scheme 1: iv; Scheme 2 and Scheme 3). As shown in scheme 3 the alkylation of the acridine derivative 27 preferentially resulted in the N-alkylated derivative 28. The O-alkylated compound could not be isolated.
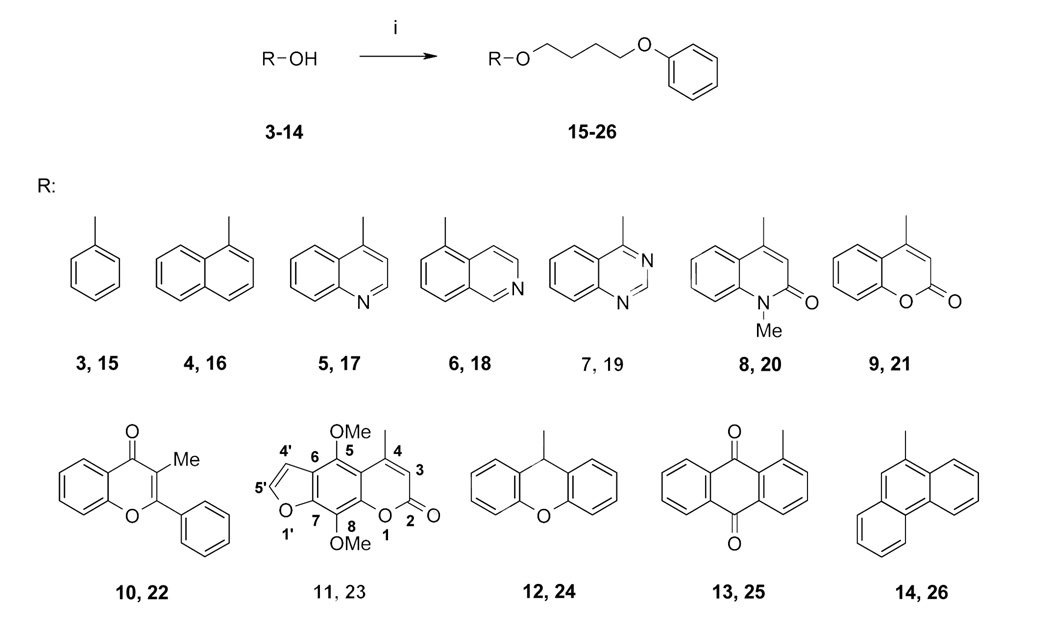
Scheme 2.
Synthesis of 4-phenoxybutoxy substituted aromatic systems using commercially available alcohols as starting compounds. (Numbers 3–14 correspond to starting compounds and numbers 15–26 to products).
Reagents: (i) elemental sodium in ethanol, 4-phenoxybutyl bromide (4-PBB) (reflux); 4-PBB, CsF-celite in CH3CN (reflux) or 4-PBB, K2CO3/KI in acetone (reflux) or DMF (120 °C or reflux).
Scheme 3.
Synthesis of compound 28.
Reagents: (i) 4-PBB and CsF-celite in CH3CN (reflux).
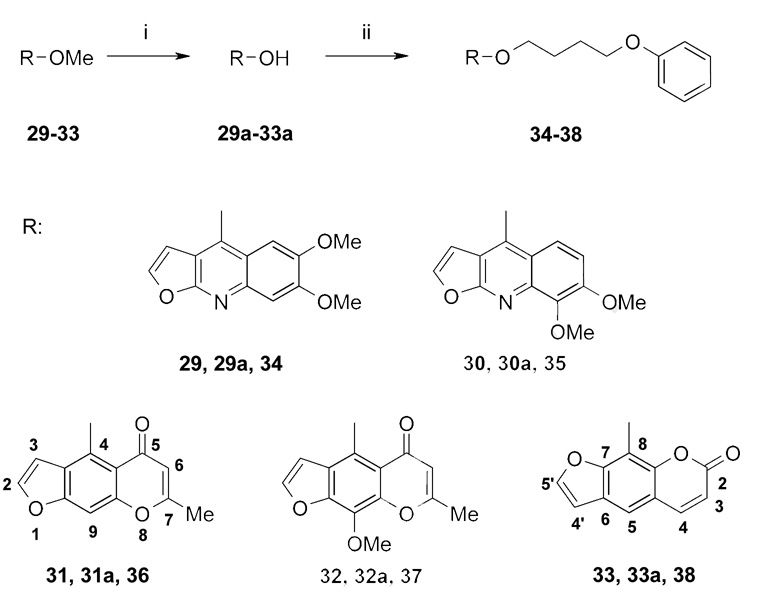
In cases where only methoxy substituted scaffolds were commercially available, we first demethylated the scaffold using either HCl or the Lewis acid boron tribromide and then re-alkylated the resulting phenols with 4-phenoxybutyl bromide (4-PBB) (Scheme 1: iii; Scheme 4). Since the methoxy substituted furoquinolines kokusagenine 29 and skimmianine 30 were not commercially available in sufficient quantities and their extraction is well established [21], we isolated them from dried leaves of Ruta graveolens by MPLC (for details see experimental part).
Scheme 4.
Synthesis of 4-phenoxybutoxy substituted aromatic systems using methoxy substituted scaffolds as starting compounds. (Numbers 29–33 correspond to starting compounds, numbers 29a–33a to the respective alcohols and numbers 34–38 to products).
Reagents: (i) HCl (reflux) or BBr3 (5 °C) and CH2Cl2 (reflux), (ii) 4-PBB, CsF-celite in CH3CN (reflux) or 4-PBB, K2CO3/KI in acetone (reflux) or DMF (120 °C or reflux).
If neither the alcohol nor the methoxy substituted derivative of a desired structure was commercially available, we built the heterocyclic ring systems in various multi-step reactions (Scheme 1: i and ii; Scheme 5–Scheme 8).
Scheme 5.
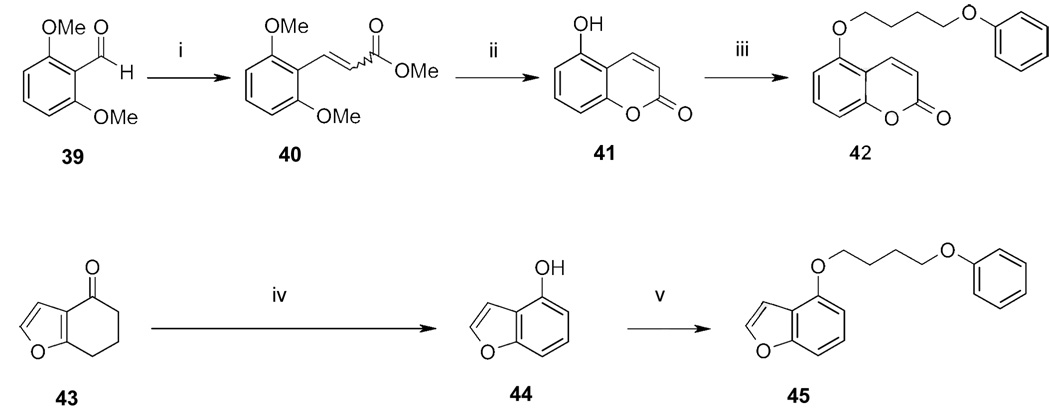
Synthesis of compounds 42 and 45.
Reagents: (i) (C6H5)3PCHCOOCH3, toluene (reflux, 3h); (ii) BBr3 (5 °C) and CH2Cl2 (reflux); (iii) 4-PBB, CsF-Celite in CH3CN (reflux); (iv) palladium-charcoal, 1-dodecene/decalin (reflux, 20 h); (v) 4-PBB, K2CO3/KI in DMF (reflux).
Scheme 8.
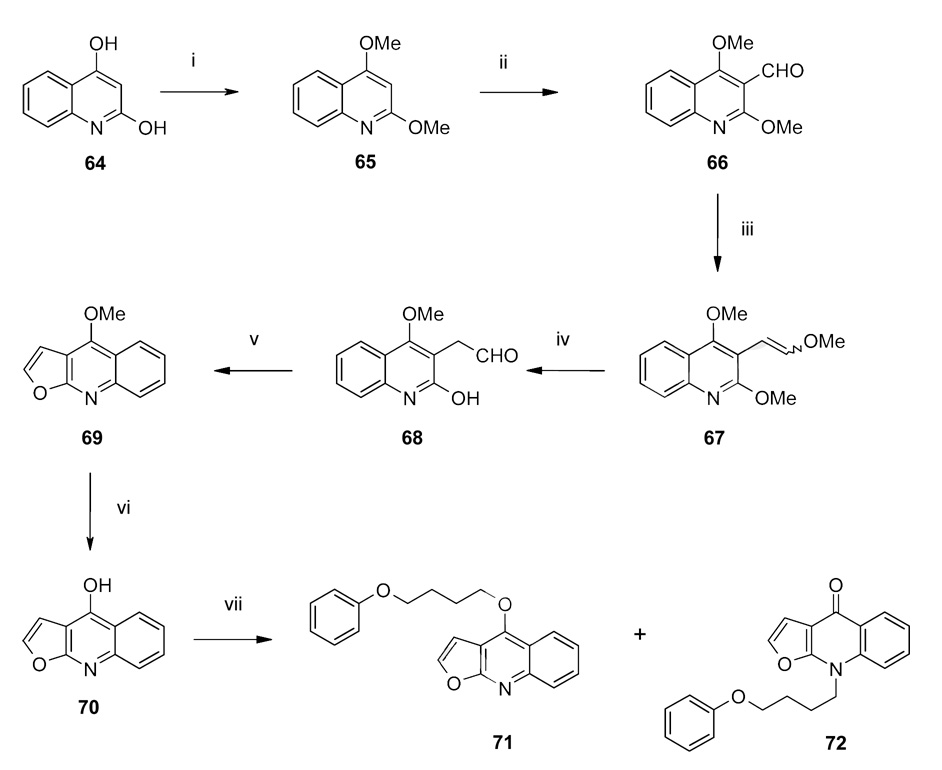
Synthesis of compounds 71 and 72.
Reagents: (i) silver carbonate in anhydrous hexane, iodomethane (room temperature, dark, 3 days); (ii) n-butyllithium (in hexanes) (−10 °C, 45 min) in anhydrous diethylether, anhydrous DMF in diethylether (0 °C for 30 min, room temperature for 45 min); (iii) potassium tert-butoxide in THF (1M), methoxymethyltriphenylphosphonium chloride in anhydrous diethylether (room temperature, 1 h), 24 h; (iv) HCl (5.5 M, refluxed, 45 min); (v) polyphosphoric acid (125 °C, 5 h); (vi) isopropylthiol, sodium hydride in anhydrous DMF (10 min, reflux, 4 h), HCl (2 M, 0 °C); (vii) KHCO3, 4-PBB in 2-butanone (reflux, 25 h).
The coumarin derivative 42 (Scheme 5) was synthesized starting from 2,6-dimethoxybenzaldehyde (39), which was reacted in a Wittig reaction to the methoxycinnamate 40. In a subsequent reaction with boron tribromide 40 was then demethylated in 5-position under simultaneous ring closure to the 5-hydroxycoumarin (41). In a final Williamson ether synthesis analogous reaction using CsF-celite as base 41 was reacted to 42 with 4-PBB. The benzofuran derivative 45 (Scheme 5) was synthesized in two steps: First, 6,7-dihydro-5H-benzofuran-4-one (43) was converted into the aromatic 4-hydroxybenzofuran (44) using Pd-charcoal as catalytic agent and 1-dodecene as hydrogen acceptor. In a second step 44 was alkylated with 4-PBB in a Williamson ether synthesis analogous reaction using potassium carbonate as base.
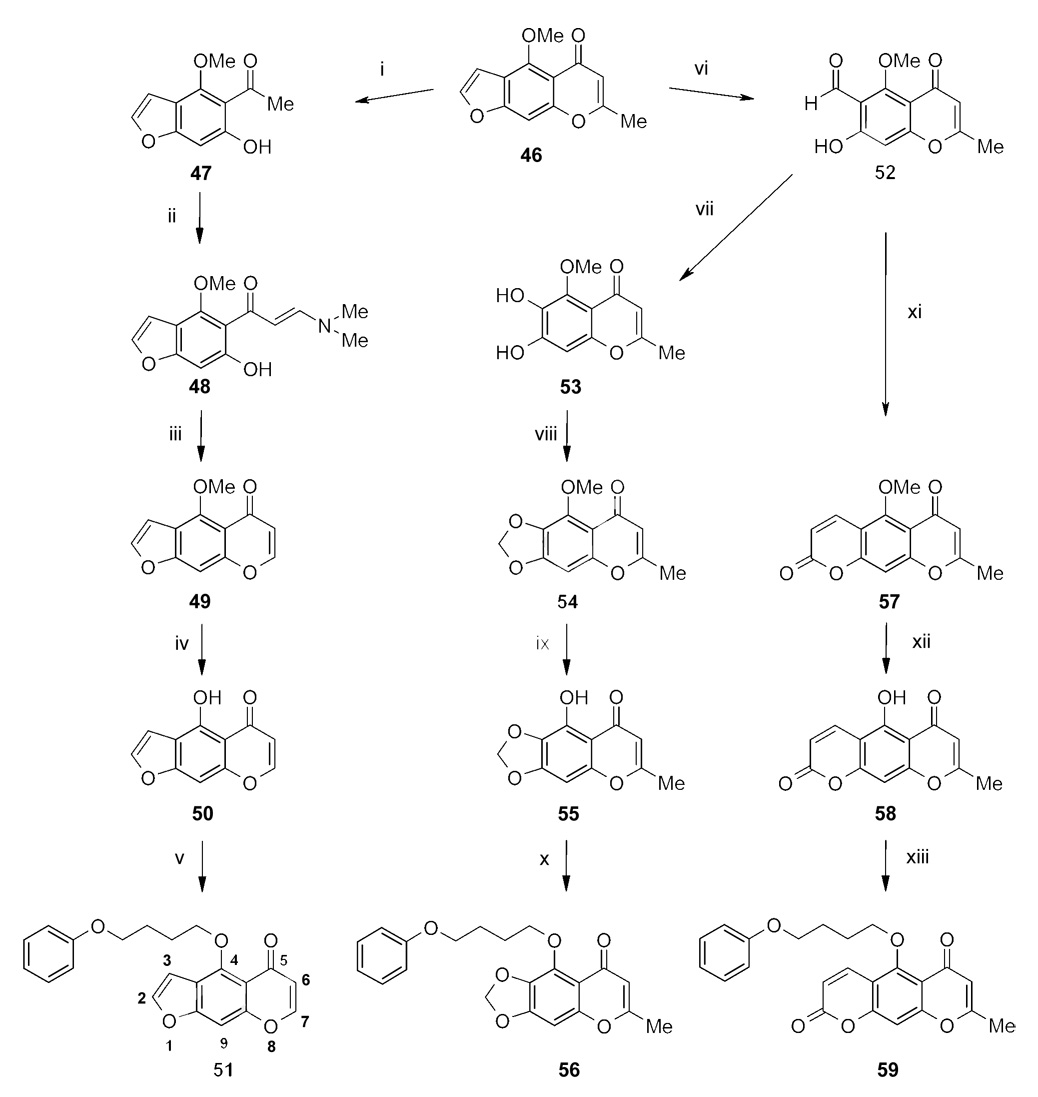
The chromone derivatives 51, 56 and 59 were synthesized according to scheme 6 using the natural product visnagin (46) as starting compound. For compound 51 the chromone ring of 46 was cleaved under basic conditions to afford the methylketone visnaginone (47). In a second step 47 was reacted with N,N-dimethylformamide dimethylacetal (DMFDMA) in an aldol condensation to give 48. The enaminone 48 was refluxed with glacial acetic acid to again form a chromone ring in an addition-elimination reaction (49). The chromone 49 was then demethylated with boron tribromide to the phenol 50 and afterwards alkylated using CsF-celite as base to afford 51. For the synthesis of compounds 56 and 59 the furan ring of visnagin (46) was oxidatively cleaved with sulfuric acid to afford 52. To obtain compound 56 the intermediate 52 was reduced to 53, afterwards alkylated with dibromomethane to 54 and then demethylated with boron tribromide to the phenol 55. In a final step, 55 was alkylated with 4-PBB in the presence of potassium carbonate to render the ether 56. For compound 59 the intermediate 52 was reacted in a Wittig reaction followed by an intramolecular lactone ring formation. The resulting coumarin ring substituted chromone 57 was then demethylated using boron tribromide and afterwards alkylated using potassium carbonate to give 59.
Scheme 6.
Synthesis of chromone derivatives 51, 56 and 59.
Reagents: (i) KOH (0.2 M; reflux, 15 min); (ii) DMFDMA in toluene (reflux, 3 h); (iii) CH3COOH (reflux, 5 h); (iv) BBr3 (5 °C) and CH2Cl2 (reflux); (v) 4-PBB, CsF-celite in CH3CN (reflux); (vi) H2SO4 (1 M), K2Cr2O7 (0.3 M, 2 h); (vii) NaOH (1 M), H2O2 (10 M), 25 °C (2–3 h); (viii) K2CO3, CH2Br2 in DMF (95 °C, 4 h); (ix) BBr3 (5 °C) and CH2Cl2 (reflux); (x) 4-PBB, K2CO3/KI in DMF (reflux); (xi) (C6H5)3PCHCOOCH3, toluene (reflux, 6 h); (xii) BBr3 (5 °C) and CH2Cl2 (reflux); (xiii) 4-PBB, K2CO3/KI in DMF (120°C).
Compound 63 was synthesized starting from khellinone (60) according to scheme 7. Coumpound 60 was first reacted in a Wittig reaction to the furocoumarin 61, which was then cleaved to the corresponding phenol 62 with boron tribromide. To obtain 63, compound 62 was alkylated as described above for the chromone derivatives.
Scheme 7.
Synthesis of compound 63.
Reagents: (i) (C6H5)3PCHCOOCH3, toluene (reflux, 3h); (ii) BBr3 (5 °C) and CH2Cl2 (reflux); (iii) 4-PBB and CsF-celite in CH3CN (reflux).
Since the furoquinoline dictamnine (69) was commercially available only in milligram quantities and furthermore too high-priced for a synthetic starting compound, we synthesized it in a 6-step reaction starting from 2,4-quinolinediol (64) according to scheme 8. The treatment of 64 in anhydrous benzene with methyl iodide in the presence of silver carbonate afforded montanine (65). Using n-butyllithium a formyl group was next introduced in 3-position of the quinoline system (66). After a Wittig reaction (67) and subsequent hydrolysis with HCl 68 was obtained. Treatment of 68 with polyphosphoric acid at 125 °C resulted in the formation of a furan ring to render the furoquinoline dictamnine (69). Surprisingly the cleavage of the methoxy group on the C-4 carbon of 69 proved difficult. Neither boron tribromide nor HCl, both commonly used reagents for demethylation reactions and used for the other herein synthesized compounds, failed to cleave the methoxy group. Finally, refluxing 69 with sodium isopropyl thiolate in DMF gave 70 in low but adequate yields. The alkylation of 70 with 4-PBB resulted in two main products, the O-alkylated (71, 27% yield) and the N-alkylated (72, 5% yield) derivative, which were separated by flash chromatography.
The thio-analog of PAP-1, 4-(4-phenoxybutoxy)-7H-furo[3,2-g]chromene-7-thione (73), was synthesized in a one step reaction adding Lawesson’s reagent to a solution of PAP-1 (Scheme 9). The preceding synthesis of PAP-1 was performed as described by Schmitz et al. [17] starting from 5-MOP.
Scheme 9.
Synthesis of compound 73.
Reagents: (i) Lawesson’s reagent in anhydrous toluene (reflux, 20 h).
3. Results and discussion
3.1. Inhibition of Kv1.3 channels
The inhibition of Kv1.3 channels by the newly synthesized compounds was determined by manual whole-cell patch-clamp on L929 cells stably expressing Kv1.3. The results are listed in Table 1. We first investigated whether simple aromatic carbocyclic systems such as phenol, 1-naphthol and 9-hydroxyphenanthrene could be turned into potent Kv1.3 blockers by attaching the 4-phenoxybutoxy side-chain. All three derivatives (compounds 15, 16 and 26) exhibited IC50 values higher than 10 µM demonstrating that the psoralen moiety of the phenoxyalkoxypsoralen-type Kv1.3 blockers cannot be replaced by 1-, 2- or 3-membered ring systems without heteroatoms. We next attached the 4-phenoxybutoxy side-chain to bicyclic ring systems containing nitrogen as a heteroatom and bi- or tricyclic ring systems containing either only oxygen or oxygen and nitrogen. The bicyclic compounds containing nitrogen like the quinoline derivative 17 and the quinazoline derivative 19 were ineffective at a concentration of 10 µM, whereas the isoquinoline derivative 18 blocked Kv1.3 with an IC50 of 520 nM. The bicyclic flavone derivative 22 containing oxygen as a heteroatom blocked Kv1.3 with an IC50 of 1 µM. The tricyclic compounds 24 and 25, both containing oxygen, were only very weak Kv1.3 blockers with IC50 values of 12 and 7 µM. Both compounds were not fully aromatic and in case of 24 not planar suggesting that aromaticity and planarity are required for high affinity Kv1.3 inhibition. The bicyclic quinolinone derivative 20, containing both nitrogen and oxygen and being an aza-analogous derivative of the coumarin system, which is part of the psoralen moiety, blocked Kv1.3 with an IC50 of 670 nM. Compound 28, an acridone derivative with the side-chain connected to nitrogen, showed a remarkably low IC50 of 165 nM demonstrating that there is a certain degree of flexibility in the binding site assuming that 28 binds to the same site on Kv1.3 as PAP-1.
Table 1.
IC50 values on Kv1.3 and Kv1.5
| Compounda | IC50 value in µM | Selectivity | |
|---|---|---|---|
| Kv1.3 | Kv1.5 | IC50 (Kv1.5)/IC50 (Kv1.3) | |
| 5-MOP [14] | 101 | 177 | 1.75 |
| PAP-1 [17] | 0.002 | 0.045 | 22.5 |
| 15 | 20 | n.d. | / |
| 16 | 15 | n.d. | / |
| 17 | > 10 | 3.5 | < 0.35 |
| 18 | 0.52 | 0.52 | 1 |
| 19 | no effectb | ~ 25 | / |
| 20 | 0.67 | 1.5 | 2.24 |
| 21 | 0.89 | 1.5 | 1.69 |
| 22 | 1 | 10 | 10 |
| 23 | 0.45 | 0.85 | 1.89 |
| 24 | 12 | 15 | 1.25 |
| 25 | 7 | 10 | 1.43 |
| 26 | no effectb | no effectb | / |
| 28 | 0.165 | 0.45 | 2.73 |
| 34 | 0.15 | 0.48 | 3.2 |
| 35 | 0.2 | 0.98 | 4.9 |
| 36 | 0.233 | 0.31 | 1.33 |
| 37 | 1.5 | 2.5 | 1.67 |
| 38 | 1 | 1 | 1 |
| 42 | 0.15 | 0.52 | 3.47 |
| 45 | 0.65 | 1 | 1.54 |
| 51 | 0.17 | 0.2 | 1.18 |
| 56 | 5 | 1.7 | 0.34 |
| 59 | 6 | 6 | 1 |
| 63 | 0.318 | 0.28 | 0.88 |
| 71 | 1 | 2 | 2 |
| 72 | 1 | 2 | 2 |
| 73 | 0.017 | 0.38 | 22 |
Each compound was tested three times at 4–5 concentrations. Each experiment was performed on a different cell.
Standard deviations: 3–20 % (Kv1.3); 3–30 % (Kv1.5).
Tested at 10 µM.
n.d. – not determined
We further synthesized a number of furoquinolines because kokusagenine (29), a furoquinoline occurring in Ruta graveolens, had been previously reported by our group to inhibit Kv1.3 with an IC50 of 10 µM [22]. Since this IC50 was lower than the IC50 of 5-MOP (1, IC50 101 µM [14]), we hoped that the 4-phenoxybutoxy substituted compound would be as potent or even more potent than PAP-1 (2, IC50 2 nM). However, the dictamnine derivative 71 and the N-alkylated compound 72 only showed IC50 values of 1 µM, whereas the dimethoxy substituted kokusagenine derivative 34 and the skimmianine derivative 35 blocked Kv1.3 with IC50 values of 150 nM and 200 nM, respectively.
As neither attachment of the 4-phenoxybutoxy side-chain to simple carbo- or heterocyclic aromatic systems rendered low nanomolar Kv1.3 blockers, we concluded that the scaffold has to be more similar to the psoralen system of PAP-1. We therefore next investigated which parts of the psoralen system were necessary for high affinity Kv1.3 blockade and which parts, if any, could be dispensed with. Since the psoralen (= furocoumarin) ring system contains both a benzofuran and a coumarin system as substructures, we attached the 4-phenoxybutoxy side-chain to both these scaffolds. The benzofuran derivative 45, which compared to the psoralen lacks only the lactone ring, was potent with an IC50 of 650 nM. The coumarin derivatives 21 and 42, lacking the furan ring of the psoralen system, showed IC50 values of 890 nM, when the side-chain was attached in the 4-position, and of 150 nM, when the side-chain was attached in the 5-position. However, since both the benzofurans and the coumarins were 100-fold less potent than the psoralen PAP-1, we next decided to look at even smaller variations on the psoralen scaffold. For the psoralen derivatives 23, 38 and 63 the position of the side-chain was varied from 5-position to 4- and 8-position and additional groups were added like methoxy groups in 5- and 8-position or a methyl group in 4-position. Compound 38, which contained the side-chain in 8-position and no further variations compared to PAP-1, showed an IC50 value of 1 µM. Compound 63 with the side-chain in 8-position and additional groups, a methoxy group in 5-position and methyl group in 4-position, and compound 23 with the side-chain in 4-position and two additional methoxy groups in 5- and 8-position exhibited IC50s of 318 nM and 450 nM, respectively. As further variations of the psoralen system we next exchanged the lactone ring (2-chromone ring) with the isomeric 4-chromone ring, which resulted in a quite potent Kv1.3 blocker (51, 170 nM). Adding a methyl group in 7-position of 51 (corresponding to the 2-position of the psoralen system) only slightly decreased potency (36, 233 nM), while further substitution with a methoxy group in 9-position (corresponding to the 8-position of the psoralen system) decreased the IC50 into the micromolar range (37, 1.5 µM). An exchange of the furan ring with either a methylenedioxy group or a 2-chromone ring reduced potency even further (56, 5 µM; 59, 6 µM). Based on these results we concluded that both the position of the side-chain as well as the substitution pattern of the psoralen system is crucial for obtaining low nanomolar Kv1.3 inhibitors. It further is not possible to exchange the 2-chromone ring for a 4-chromone ring without significantly loosing potency.
Since every greater variation of the psoralen scaffold resulted in a significant loss of potency in comparison to PAP-1 (IC50 2 nM), we next decided to only replace one atom in the psoralen scaffold and exchanged the carbonyl oxygen in 2-position with a sulfur. This variation resulted in 73 (IC50 17 nM), the most potent Kv1.3 blocker in our current study. HPLC studies on stability showed that 73 is stable in DMSO stock solutions for more than 12 months and in Ringer solution for at least 24 hours (data not shown). This demonstrates that the Kv1.3 inhibition measured in the electrophysiological experiments is due to 73 itself and not to PAP-1 resulting from potential hydrolysis.
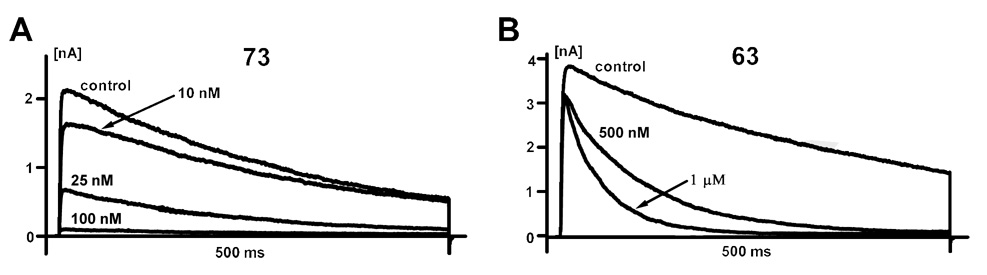
All our newly synthesized compounds exhibited Hill coefficients close to 2 for Kv1.3 inhibition similar to the psoralens Psora-4 and PAP-1 [16,17], 7-substituted khellinones [23], khellinone chalcones [24] and the cyclohexyl-substituted benzamides (cyclohexanones) [25], suggesting that two or more molecules of each compound interact with one Kv1.3 channel protein in a cooperative manner. However, the synthesized compounds differed in their blocking kinetics (Figure 2). Compounds 34, 35, 36 and 73 exhibited pronounced “use-dependence” (meaning that these compounds only reach equilibrium block after 8 to 10 depolarizing pulses) indicating a preferential block of the C-type inactivated state of Kv1.3 similar to what had been previously described for the psoralens Psora-4 and PAP-1 [16,17] (Figure 2A). In contrast, the remaining compounds exhibited a typical open channel block (Figure 2B) similar to verapamil and the khellinone chalcones [24] and could be washed out easily during the patch-clamp experiments.
Figure 2.
Kinetics of Block. The synthesized compounds can be divided into two groups depending on their kinetics of block: A) Compounds 34, 35, 36 and 73 (shown) block the C-type inactivated state. B) All other compounds (63 shown as an example) are open-channel blockers.
3.2. Selectivity over Kv1.5 channels
In addition to testing the newly synthesized compounds on Kv1.3, we determined their selectivity over the closely related Kv1.5 channel. Cross-reactivity to the cardiac potassium channel Kv1.5 is a common problem for small molecule Kv1.3 blockers. Nearly all known small molecule Kv1.3 blockers such as correolide [26], PAC [25], Psora-4 [16] and the khellinone chalcones [24] inhibit Kv1.5 as potently as Kv1.3 or only display a very moderate 2–3 fold selectivity. Out of the existing small molecule Kv1.3 blockers, our template PAP-1 currently displays the highest selectivity (23-fold) for Kv1.3 over Kv1.5 [17].
As shown in Table 1, the selectivity, which we define as IC50 for Kv1.5 divided by the IC50 for Kv1.3, ranged from 0.34-fold for compound 17 to 22-fold for compound 73. While compounds 17, 63 and 56 inhibited Kv1.5 roughly 3-times more potently than Kv1.3, most compounds were relatively unselective and inhibited both channels with nearly equal affinity or showed a moderate 2–5 selectivity for Kv1.3. The only compound that exhibited a higher selectivity is the thio-analog of PAP-1, compound 73, which is 22-times more potent on Kv1.5 and hence as selective as PAP-1.
4. Conclusions
We here synthesized a series of 4-phenoxybutoxy substituted heterocycles with the aim of obtaining alternative Kv1.3 inhibitors that do not contain the psoralen system of our previously described Kv1.3 blocker PAP-1. A high percentage of these newly synthesized compounds such as 4-phenoxybutoxy substituted quinolinones, furoquinolines, coumarins or furochromones, blocked Kv1.3 with IC50 values between 150 nM to 10 µM. Especially compound 42, which lacks only the furan ring of PAP-1, is a relatively potent Kv1.3 blocker (IC50 = 150 nM). In contrast, compounds where the psoralen system was replaced by quinolines, quinazolines or simple carbocyclic aromatic systems such as phenanthrene had no effect on Kv1.3 demonstrating that attaching a 4-phenoxybutoxy side-chain is not sufficient to render any aromatic system a potent Kv1.3 blockers. In general, all variations of the PAP-1 structure significantly reduced Kv1.3 blocking potency demonstrating that both the psoralen system and the 4-phenoxybutoxy side-chain are essential for potent Kv1.3 inhibition. The only structural variation that was tolerated in the pharmacophore was a replacement of the carbonyl oxygen in the coumarin ring of the psoralen system with a sulfur atom (73, IC50 17 nM). Despite the fact that we were not able to obtain a more potent Kv1.3 blocker than our previously published compound PAP-1, the newly synthesized compounds will be valuable tool compounds for validating mutagenesis and/or molecular modeling studies that are aiming to define the binding site of PAP-1 on the Kv1.3 protein.
Based on our results we conclude that phenoxyalkoxypsoralen-type Kv1.3 blockers have a very steep structure-activity relationship and that PAP-1 very likely already constitutes the optimum in terms of potency and selectivity that is attainable with this pharmacophore. However, in future it will be interesting to further probe the pharmacophore with subtle variations such as replacing more than one of the oxygen atoms in the psoralen system with nitrogen or sulfur.
5. Experimental sections
5.1. Material and methods
Melting points were determined in open capillary tubes using an electrothermal melting point apparatus (Stuart Scientific, SMP03) and are uncorrected. Mass spectra (MS) were recorded on a Hewlett-Packard HP5989A-MS Engine mass spectrometer (70 eV, low resolution) or a Finnigan MAT 8230 (70 eV, high resolution) at the University of Kiel (Germany) or on a VG ProSpec spectrometer (70 eV, low and high resolution) at the University of California, Berkeley. Elemental analyses were performed by a Hewlett-Packard CHN autoanalyzer for carbon, nitrogen, hydrogen, and sulfur at the University of Kiel, Germany. Proton nuclear magnetic resonance (1H NMR) and carbon nuclear magnetic resonance (13C NMR) were performed on a Bruker ARX 300 (University of Kiel, Germany) or on a Bruker ARX 500 (University of California, Davis) with the mentioned solvents. Chemical shifts are reported in parts per million (ppm) on the δ scale and were referenced to the appropriate solvent peaks (CDCl3 referenced to CHCl3 at δH 7.24 ppm; DMSO-d6 referenced to DMSO at 2.49 ppm) or TMS as internal standard. If necessary the 1H and 13C peaks were assigned with the help of COLOC, COSY and NOESY experiments. Signals are designated as follows: s (singlet), d (doublet), dd (doublet of doublets), ddd (doublet of doublet of doublets), dt (doublet of triplets), t (triplet), quint (quintet), m (multiplet). Yields were not optimized. Analytical thin-layer chromatography (TLC) was performed on Merck silica gel 60F254 aluminum-backed plates. Chromatographic separation was performed by flash (Merck silica gel, 40–60 µm) or middle pressure chromatography (MPLC) (LiChroprep® Merck 25–40 µm and 40–63 µm or aluminum oxide 90 active, basic, 63–200 µm). The proportions of solvents for the chromatography are expressed in volume to volume (v:v). As described in the chemistry section commercially available alcohols were used directly for a Williamson ether synthesis or an analogous synthesis (general procedure A or B, scheme 2 and scheme 3). Commercially available methoxy substituted starting compounds were demethylated according to general procedure C (scheme 4). All non-commercially available starting compounds were synthesized (scheme 5–scheme 9).
5.2. General Synthesis Procedure
5.2.1. Williamson analog ether synthesis
5.2.1.1. General procedure A
To a suspension of CsF-celite (preparation of CsF-celite see Lee et al. [27]) and the respective alcohol in dried acetonitrile the alkylating agent, 4-phenoxybutyl bromide (4-PBB), was added dropwise and the reaction mixture was refluxed. The progress of the reaction was monitored by TLC. After completion of the reaction, the reaction mixture was filtered and the solvent was evaporated in vacuo. The resulting residue was purified by MPLC and/or recrystallized.
5.2.1.2. General procedure B
The respective alcohol was dissolved in acetone or DMF. K2CO3 and the alkylating agent, 4-PBB, were added. Afterwards the mixture was refluxed in the presence of a catalytic amount of KI, if necessary under nitrogen atmosphere. The progress of the reaction was monitored by TLC. After completion of the reaction, the reaction mixture was filtered and the solvent was evaporated in vacuo. The resulting residue was dissolved in CH2Cl2 and extracted three times with NaOH (0.5 M). The pooled organic phases were dried over Na2SO4 and concentrated in vacuo. The resulting residue was purified by MPLC or recrystallized to give the pure compound.
5.2.2. Demethylation
5.2.2.1. General procedure C
The methoxy substituted starting compound was dissolved in CH2Cl2 and cooled with an ice bath. Boron tribromide was slowly added so that the temperature remained below 5 °C. After the addition was completed the reaction mixture was refluxed for 30 min to 4 h and then quenched with water. The aqueous phase was extracted twice with CH2Cl2 and afterwards the pooled organic phases were evaporated. The resulting residue was purified by recrystallization or MPLC to obtain the pure alcohols.
5.3. Synthesized Compounds
5.3.3. 1,1'[1,4-Butanediyl(oxy)]bis-benzene (15)
350 mg (15 mmol) of sodium were dissolved in 80 mL ethanol and 1.98 g of phenol (3, 21 mmol) were added. After subsequently adding 2.75 g of 4-PBB (12 mmol) and refluxing the solution for 2 h the solvent was evaporated and NaOH (1.5 M) was added to the resulting residue. This solution was extracted twice with diethylether. The pooled organic phases were washed with water, dried over Na2SO4 and evaporated under vacuum. After recrystallization from ethanol 15 (1.5 g, 30%) was obtained as white crystals: Mp 101 °C (Lit. 101 °C [28]). The spectroscopic data were in accordance with literature [28].
5.3.4. 1-(4-Phenoxybutoxy)naphthalene (16)
350 mg (15 mmol) of sodium were dissolved in 80 mL ethanol and 3.03 g of 1-naphthol (4, 21 mmol) were added. After adding 2.75 g of 4-PBB (12 mmol) and refluxing the solution for 2 h the solvent was evaporated and NaOH (1.5 M) was added to the resulting residue. This solution was extracted twice with diethylether. The pooled organic phases were washed with water, dried over Na2SO4 and evaporated under vacuum to give 16 (550 mg, 9%) as white crystals: Mp 76–77 °C; 1H NMR (DMSO-d6) δ: 1.95-2.04 (m, 4H, 4-O-CH2-(CH2)2-CH2-O-C6H5), 4.08 (t, 2H, 3J = 6.1 Hz, 4-O-(CH2)3-CH2-O-C6H5), 4.22 (t, 2H, 3J = 5.9 Hz, 4-O-CH2-(CH2)3-O-C6H5), 6.91-6.97 (m, 4H, H-4, H-2', H-4' and H-6'), 7.26-7.30 (m, 2H, H-3' and H-5'), 7.41 (t, 1 H, 3J = 7.9 Hz, H-3), 7.45-7.53 (m, 3H, H-2, H-6, H-7), 7.86 (d, 1H, 3J = 7.95 Hz, H-5), 8.15 (d, 1H, 3J = 8.3 Hz, H-8); MS (EI) m/z 292 [M+] (12), 149 (81), 127 (10), 115 (24), 107 (100), 94 (6), 77 (37), 65 (7), 55 (31); Anal. calcd for C20H20O2: C, 82.16; H, 6.89; found: C, 82.46; H, 7.01.
5.3.5. 4-(4-Phenoxybutoxy)quinoline (17)
4-Hydroxyquinoline (5, 590 mg, 4.1 mmol), CsF-celite (1.3 g, 6.1 mmol) and 4-PBB (1.87 g, 8.2 mmol), dissolved in 5 mL of dried acetonitrile, were reacted according general procedure A. The crude product was purified by MPLC (cyclohexane/ethyl acetate/methanol (11:9:1)) to give 17 (354 mg, 30%) as a white solid: Mp 66.5 °C; 1H NMR (DMSO-d6) δ: 1.94-2.08 (m, 4H, 4-O-CH2-(CH2)2-CH2-O-C6H5), 4.07 (t, 2H, 3J = 6.1 Hz, 4-O-(CH2)3-CH2-O-C6H5), 4.30 (t, 2H, 3J = 5.9 Hz, 4-O-CH2-(CH2)3-O-C6H5), 6.89-6.95 (m, 3H, H-2', H-4' and H-6'), 7.00 (d, 1H, 3J = 5.2 Hz, H-3), 7.25-7.30 (m, 2H, H-3' and H-5'), 7.52 (ddd, 1H, 3J = 8.2 Hz, 3J = 6.9 Hz, 4J = 1.2 Hz, H-6), 7.72 (ddd, 1H, 3J = 8.4 Hz, 3J = 6.9 Hz, 4J = 1.5 Hz, H-7), 7.95 (d, 1H, 3J = 8.3 Hz, H-8), 8.13 (dd, 1H, 3J = 8.3 Hz, 4J = 0.9 Hz, H-5), 8.72 (d, 1H, 3J = 5.2 Hz, H-2); 13C NMR (DMSO-d6) δ: 25.08, 25.43, 66.85, 67.98, 101.46, 114.40, 120.38, 120.74, 121.45, 125.55, 128.59, 129.40, 129.59, 148.68, 151.54, 158.53, 160.62; MS (EI) m/z 293 [M+] (23), 200 (14), 158 (21), 149 (100), 128 (12), 107 (99), 94 (13), 77 (38), 65 (7), 55 (50); Anal. calcd for C19H19NO2: C, 77.79; H, 6.53; N, 4.77; found: C, 77.89; H, 6.75; N, 4.78.
5.3.6. 5-(4-Phenoxybutoxy)isoquinoline (18)
5-Hydroxyisoquinoline (6, 1.0 g, 6.89 mmol), 4-PBB (2.37 g, 10.35 mmol) and K2CO3 (2.75 g,19.93 mmol) in 35.5 mL of dried DMF were reacted according to general procedure B. The crude product was purified by MPLC (cyclohexane/ethyl acetate/methanol (30:9:1)) to give 18 (1.89 g, 94%) as a light yellow solid: Mp 80.5 °C; 1H NMR (DMSO-d6) δ: 1.92-2.08 (m, 4H, 4-O-CH2-(CH2)2-CH2-O-C6H5), 4.08 (t, 2H, 3J = 6.0 Hz, 4-O-(CH2)3-CH2-O-C6H5), 4.25 (t, 2H, 3J = 5.8 Hz, 4-O-CH2-(CH2)3-O-C6H5), 6.89-6.95 (m, 3H, H-2', H-4' and H-6'), 7.21-7.30 (m, 3H, H-7, H-3', H-5'), 7.58 (t, 1H, 3J = 7.9 Hz, H-6 or H-8), 7.63 (t, 1H, 3J = 8.5 Hz, H-6 or H-8), 7.92 (d, 1H, 3J = 5.8 Hz, H-3), 8.47 (d, 1H, 3J = 5.8 Hz, H-4), 9.26 (s, 1H, H-1); 13C NMR (DMSO-d6) δ: 25.25, 25.48, 66.90, 67.72, 109.08, 114.33, 114.39, 118.91, 120.33, 127.44, 127.79, 128.91, 129.35, 142.46, 151.67, 152.99, 158.51; MS (EI) m/z 293 [M+] (6), 149 (64), 117 (6), 116 (6), 107 (100), 94 (9), 77 (41), 65 (7), 55 (57), 41 (9); HRMS calcd for C19H20NO2 [M+H]+ 294.1494, found 294.1480.
5.3.7. 4-(4-Phenoxybutoxy)quinazoline (19)
4-Hydroxyquinazoline (7, 315 mg, 2.16 mmol), CsF-celite (1.92 g, 9.0 mmol) and 4-PBB (945 mg, 4.12 mmol), dissolved in 5 mL of dried acetonitrile, were reacted according to general procedure A. The crude product was purified by MPLC (cyclohexane/ethyl acetate/methanol (32:8:1)) to give 19 (352 mg, 55%) as a white solid: Mp 130 °C; 1H NMR (DMSO-d6) δ: 1.70-1.79 (m, 2H) and 1.82-1.92 (m, 2H) (4-O-CH2-(CH2)2-CH2-O-C6H5), 3.99 (t, 2H, 3J = 6.2 Hz, 4-O-(CH2)3-CH2-O-C6H5), 4.06 (t, 2H, 3J = 7.0 Hz, 4-O-CH2-(CH2)3-O-C6H5), 6.87-6.92 (m, 3H, H-2', H-4' and H-6'), 7.22-7.28 (m, 2H, H-3' and H-5'), 7.54 (ddd, 1H, 3J = 8.1 Hz, 3J = 7.7 Hz, 4J = 1.1 Hz, H-7), 7.68 (dd, 1H, 3J = 8.2 Hz, 4J = 0.5 Hz, H-5), 7.82 (ddd, 1H, 3J = 8.4 Hz, 3J = 7.3 Hz, 4J = 1.5 Hz, H-6), 8.17 (dd, 1H, 3J = 9.4 Hz, 4J = 1.5 Hz, H-8), 8.42 (s, 1H, H-2); 13C NMR (DMSO-d6) δ: 25.46, 25.80, 45.59, 66.80, 114.37, 120.35, 121.50, 125.94, 126.88, 127.08, 129.33, 134.10, 147.89, 147.92, 158.46, 160.12; MS (EI) m/z 294 [M+] (5), 201 (100), 159 (38), 147 (42), 129 (26), 102 (11), 94 (12), 77 (29), 55 (82), 41 (17); Anal. calcd for C18H18N2O2: C, 73.45; H, 6.16; N, 9.52; found: C, 73.52; H, 6.24; N, 9.40.
5.3.8. 1,2-Dihydro-N-methyl-4-(4-phenoxybutoxy)quinolin-2-one (20)
1,2-Dihydro-4-hydroxy-N-methylquinolin-2-one (8, 430 mg, 2.45 mmol), CsF-celite (1.89 g, 8.92 mmol) and 4-PBB (1.67 g, 7.27 mmol), dissolved in 5 mL of dried acetonitrile, were reacted according to general procedure A. The crude product was purified by MPLC (cyclohexane/ethyl acetate/methanol (32:8:1)) to give 20 (130 mg, 16%) as a white solid: Mp 80 °C; 1H NMR (DMSO-d6) δ: 1.88-2.04 (m, 4H, 4-O-CH2-(CH2)2-CH2-O-C6H5), 3.55 (s, 3H, N-CH3), 4.06 (t, 2H, 3J = 6.0 Hz, 4-O-(CH2)3-CH2-O-C6H5), 4.19 (t, 2H, 3J = 5.8 Hz, 4-O-CH2-(CH2)3-O-C6H5), 6.02 (s, 1H, H-3), 6.89-6.95 (m, 3H, H-2', H-4' and H-6'), 7.19-7.30 (m, 3H, H-7, H-3' and H-5'), 7.47 (d, 1H, 3J = 8.5 Hz, H-8), 7.63 (ddd, 1H, 3J = 8.6 Hz, 3J = 7.2 Hz, 4J = 1.5 Hz, H-6), 7.87 (dd, 1H, 3J = 8.0 Hz, 4J = 1.3 Hz, H-5); 13C NMR (DMSO-d6) δ: 24.86, 25.38, 28.48, 66.78, 68.04, 96.64, 114.38, 114.50, 115.55, 120.34, 121.36, 122.54, 129.35, 131.26, 139.38, 158.48, 160.91, 162.26; MS (EI) m/z 323 [M+] (41), 230 (26), 176 (40), 175 (29), 149 (48), 120 (33), 107 (100), 94 (22), 77 (57), 55 (82); Anal. calcd for C20H21NO3: C, 74.28; H, 6.55; N, 4.33; found: C, 74.39; H, 6.61; N, 4.31.
5.3.9. 4-(4-Phenoxybutoxy)-2H-[1]benzopyran-2-one (21)
4-Hydroxy-2H-[1]benzopyran-2-one (9, 330 mg, 2 mmol), CsF-celite (1.91 g, 9 mmol) and 4-PBB (916 mg, 4 mmol), dissolved in 5 mL of dried acetonitrile, were reacted according to general procedure A. The crude product was purified by MPLC (cyclohexane/ethyl acetate (3:1)) to give 21 (375 mg, 60%) as a white solid: Mp 79 °C; 1H NMR (DMSO-d6) δ: 1.89-2.05 (m, 4H, 4-O-CH2-(CH2)2-CH2-O-C6H5), 4.07 (t, 2H, 3J = 6.0 Hz, 4-O-(CH2)3-CH2-O-C6H5), 4.29 (t, 2H, 3J = 6.0 Hz, 4-O-CH2-(CH2)3-O-C6H5), 5.90 (s, 1H, H-3), 6.90-6.96 (m, 3H, H-2', H-4' and H-6'), 7.25-7.41 (m, 4H, H-7, H-8, H-3' and H-5'), 7.65 (ddd, 1H, 3J = 8.6 Hz, 3J = 7.3 Hz, 4J = 1.6 Hz, H-6), 7.78 (dd, 1H, 3J = 7.9 Hz, 4J = 1.5 Hz, H-5); 13C NMR (DMSO-d6) δ: 24.67, 25.21, 66.67, 69.12, 90.45, 114.36, 115.18, 116.35, 120.36, 122.73, 124.05, 129.37, 132.61, 152.69, 158.45, 161.57, 164.85; MS (EI) m/z 310 [M+] (14), 217 (29), 175 (24), 163 (30), 149 (29), 120 (41), 107 (100), 94 (31), 77 (63), 55 (100); Anal. calcd for C19H18O4: C, 73.53; H, 5.85; found: C, 73.60; H, 5.98.
5.3.10. 3-(4-Phenoxybutoxy)-2-phenyl-4H-[1]benzopyran-4-one (22)
3-Hydroxy-2-phenyl-4H-[1]benzopyranone (10, 550 mg, 2.3 mmol), CsF-celite (1.89 g, 8.9 mmol) and 4-PBB (1.42 g, 6.2 mmol), added little by little, were reacted according to general procedure A. The crude product was purified by MPLC (cyclohexane/ethyl acetate (9:1)) to give 22 (850 mg, 96%) as a white solid: Mp 66 °C; 1H NMR (DMSO-d6) δ: 1.74-1.78 (m, 4H, 3-O-CH2-(CH2)2-CH2-O-C6H5), 3.93 (t, 2H, 3J = 5.8 Hz, 3-O-(CH2)3-CH2-O-C6H5), 4.06 (t, 2H, 3J = 5.8 Hz, 3-O-CH2-(CH2)3-O-C6H5), 6.86-6.92 (m, 3H, H-2", H-4" and H-6"), 7.23-7.28 (m, 2H, H-3" and H-5"), 7.47-7.52 (m, 1H, H-6), 7.56-7.58 (m, 3H, H-2', H-4' and H-6'), 7.73 (dd, 1H, 3J = 8.3 Hz, 4J = 0.8 Hz, H-8), 7.82 (ddd, 1H, 3J = 8.5 Hz, 3J = 7.0 Hz, 4J = 1.6 Hz, H-7), 8.04-8.07 (m, 2H, H-3' and H-5'), 8.11 (dd, 1H, 3J = 7.9 Hz, 4J = 1.6 Hz, H-5); 13C NMR (DMSO-d6) δ: 25.18, 26.00, 66.77, 71.50, 114.33, 118.31, 120.26, 123.47, 124.88, 124.91, 128.32, 128.42, 129.30, 130.47, 130.70, 133.90, 139.74, 154.68, 155.27, 158.49, 173.88; MS (EI) m/z 386 [M+] (4), 237 (21), 149 (100), 121 (9), 107 (75), 94 (8), 77 (47), 65 (10), 55 (43), 51 (9); Anal. calcd for C25H22O4: C, 77.70; H, 5.74; found: C, 77.93; H, 5.86.
5.3.11. 5,8-Dimethoxy-4-(4-phenoxybutoxy)psoralen (23)
4-Hydroxy-5,8-dimethoxypsoralen (11, 793 mg, 3.02 mmol), K2CO3 (1.21 g, 8.77 mmol) and 4-PBB (1.04 g, 4.5 mmol), dissolved in 10 mL of dried DMF (120 °C), were reacted according to general procedure B. The crude product was purified by MPLC (cyclohexane/ethyl acetate (7:3)) to give 23 (260 mg, 21%) as a white solid: Mp 94.5 °C; 1H NMR (DMSO-d6) δ: 1.88-1.99 (m, 4H, 4-O-CH2-(CH2)2-CH2-O-C6H5), 3.92 (s, 3H, 5-OCH3 or 8-OCH3), 4.02-4.16 (m, 5H, 5-OCH3 or 8-OCH3 and 4-O-(CH2)3-CH2-O-C6H5), 4.23-4.30 (m, 2H, 4-O-CH2-(CH2)3-O-C6H5), 5.76 (s, 1H, H-3), 6.89-6.96 (m, 3H, H-2", H-4" and H-6"), 7.19 (d, 1H, 3J = 2.3 Hz, H-4'), 7.25-7.30 (m, 2H, H-3" and H-5"), 8.07 (d, 1H, 3J = 2.2 Hz, H-5'); 13C NMR (DMSO-d6) δ: 24.93, 25.29, 61.01, 62.10, 66.76, 69.19, 88.55, 104.90, 105.33, 114.35, 118.78, 120.32, 128.59, 129.33, 143.05, 145.10, 146.55, 147.95, 158.51, 160.92, 167.17; MS (EI) m/z 410 [M+] (34), 220 (17), 205 (16), 177 (9), 149 (82), 107 (100), 79 (12), 77 (30), 65 (6), 55 (34); Anal. calcd for C23H22O7: C, 67.31; H, 5.40; found: C, 67.02; H, 5.52.
5.3.12. 9-(Phenoxybutoxy)-9H-dibenzo[b,e]pyran (24)
9-Hydroxy-9H-dibenzo[b,e]pyran (12, 3.5 g, 20 mmol), K2CO3 (8 g, 58 mmol) and 4-PBB (5.73 g, 25 mmol dissolved in 35 mL DMF and added dropwise) were reacted in 100 mL of dried DMF were reacted according to general procedure B at 120 °C under a nitrogen atmosphere. The crude product was purified by MPLC (cyclohexane/ethyl acetate (19:1)) to give 24 (150 mg, 2%) as a slightly white crystals: Mp 96.5 °C; 1H NMR (DMSO-d6) δ: 1.46-1.66 (m, 4H, 9-O-CH2-(CH2)2-CH2-O-C6H5), 3.25 (t, 2H, 3J = 6.1 Hz, 9-O-(CH2)3-CH2-O-C6H5), 3.82 (t, 2H, 3J = 6.3 Hz, 9-O-CH2-(CH2)3-O-C6H5), 5.70 (s, 1H, H-9), 6.83 (d, 2H, 3J = 7.9 Hz, H-1 and H-8), 6.89 (t, 1H, 3J = 7.3 Hz) and 7.19-7.27 (m, 6H) (H-3, H-6, H-2', H-3', H-4', H-5' and H-6'), 7.40-7.45 (m, 2H, H-2 and H-7), 7.56 (dd, 2H, 3J = 7.5 Hz, 3J = 1.3 Hz, H-4 and H-5); 13C NMR (DMSO-d6) δ: 25.44, 25.78, 64.44, 66.82, 69.28, 114.29, 116.19, 120.24, 120.55, 123.25, 129.30, 129.60, 130.08, 151.63, 158.47; MS (EI) m/z 346 [M+] (1), 197 (29), 181 (100), 152 (8), 149 (8), 94 (16), 77 (7), 71 (7), 55 (5), 41 (5); HRMS calcd for C23H22O3 [M+] 346.1569, found 346.1552.
5.3.13. 1-(4-Phenoxybutoxy)anthraquinone (25)
1-Hydroxyantraquinone (13, 897 mg, 4 mmol), K2CO3 (1.6 g, 11.6 mmol) and 4-PBB (1.37 g, 6 mmol) in 40 mL of dried DMF (120 °C), were reacted according to general procedure B. The crude product was purified by MPLC (cyclohexane/ethyl acetate (19:1)) to give 25 (600 mg, 40%) as a yellow solid: Mp 151.5 °C; 1H NMR (DMSO-d6) δ: 1.94-2.13 (m, 4H, 1-O-CH2-(CH2)2-CH2-O-C6H5), 4.15 (t, 2H, 3J = 6.2 Hz, 1-O-(CH2)3-CH2-O-C6H5), 4.29 (t, 2H, 3J = 5.7 Hz, 1-O-CH2-(CH2)3-O-C6H5), 6.90-7.00 (m, 3H, H-2', H-4' and H-6'), 7.27-7.32 (m, 2H, H-3' and H-5'), 7.61-7.64 (m, 1H, H-2), 7.83-7.97 (m, 4H, H-3, H-4, H-7 and H-8), 8.15-8.19 (m, 2H, H-6 and H-9); 13C NMR (DMSO-d6) δ: 25.29, 25.48, 67.01, 68.68, 114.35, 118.80, 119.89, 120.23, 120.67, 125.99, 126.53, 129.29, 131.89, 133.39, 134.47, 134.92, 135.29, 158.56, 159.33, 181.01, 182.75; MS (EI) m/z 372 [M+] (1), 279 (26), 237 (9), 225 (17), 147 (8), 120 (7), 107 (14), 94 (9), 77 (19), 55 (100); Anal. calcd for C24H20O4: C, 77.40; H, 5.41; found: C, 77.60; H, 5.46.
5.3.14. 9-(4-Phenoxybutoxy)phenanthrene (26)
9-Hydroxyphenanthrene (14, 700 mg, 3.6 mmol), K2CO3 (1.49 g, 10.8 mmol) and 4-PBB (2.67 g, 11.6 mmol) in 25 mL of dried DMF (120 °C), were reacted according to general procedure B. After dissolving the resulting residue in CH2Cl2 the crude product was precipitated by adding cyclohexane/ethyl acetate (3:2) and recrystallized from ethanol to give 26 (700 mg, 57%) as pale yellow crystals: Mp 105.5 °C; 1H NMR (DMSO-d6) δ: 1.99-2.09 (m, 4H, 9-O-CH2-(CH2)2-CH2-O-C6H5), 4.09 (t, 2H, 3J = 6.0 Hz, 9-O-(CH2)3-CH2-O-C6H5), 4.31 (t, 2H, 3J = 5.8 Hz, 9-O-CH2-(CH2)3-O-C6H5), 6.90-6.96 (m, 3H, H-2', H-4' and H-6'), 7.16-7.31 (m, 3H, H-10, H-3' and H-5'), 7.47-7.74 (m, 4H, H-2, H-3, H-6 and H-7), 7.85 (dd, 1H, 3J = 7.8 Hz, 4J = 1.1 Hz, H-1 or H-8), 8.29 (dd, 1H, 3J = 8.0 Hz, 4J = 1.1 Hz, H-1 or H-8), 8.69 (d, 1H, 3J = 7.9 Hz, H-4 or H-5), 8.78 (d, 1H, 3J = 8.0 Hz, H-4 or H-5); 13C NMR (DMSO-d6) δ: 25.32, 25.63, 66.93, 67.37, 102.80, 114.39, 120.32, 121.96, 122.56, 122.80, 124.24, 126.49, 126.94, 127.19, 127.24, 125.67, 125.86, 129.35, 130.64, 132.54, 134.47, 134.92, 151.92, 158.53; MS (EI) m/z 342 [M+] (17), 194 (20), 165 (30), 149 (100), 107 (92), 95 (5), 94 (5), 79 (13), 77 (29), 65 (6), 55 (35); HRMS calcd for C24H22O2 [M]+ 342.1620, found 342.1614.
5.3.15. 9,10-Dihydro-4-methoxy-N-(4-phenoxybutyl)acridin-9-one (28)
9-Hydroxy-4-methoxyacridine (27, 450 mg, 2 mmol), CsF-celite (1.91 g, 9 mmol) and 4-PBB (1.15 g, 5.1 mmol), dissolved in 5 mL of dried acetonitrile, were reacted according to general procedure A. The crude product was purified by MPLC (cyclohexane/ethyl acetate/methanol (30:9:1)) to give 28 (180 mg, 24%) as a yellow solid: Mp 128.5 °C; 1H NMR (C6D6) δ: 1.42-1.49 (m, 2H, N-(CH2)2-CH2-CH2-O-C6H5), 1.83-1.90 (m, 2H, N-CH2-CH2-(CH2)2-O-C6H5), 3.33 (s, 3H, 4-OCH3), 3.58 (t, 2H, 3J = 6.1 Hz, N-(CH2)3-CH2-O-C6H5), 4.22 (t, 2H, 3J = 7.7 Hz, N-CH2-(CH2)3-O-C6H5), 6.74 (dd, 1H, 3J = 7.8 Hz, 4J = 1.5 Hz, H-3), 6.91 (dd, 2H, 3J = 8.7 Hz, 4J = 1.0 Hz, H-2' and H-6'), 6.97 (t, 1H, 3J = 7.4 Hz, H-4'), 7.05 (t, 1H, 3J = 7.9 Hz, H-2), 7.09 (ddd, 1H, 3J = 7.9 Hz, 3J = 6.2 Hz, 4J = 1.6 Hz, H-7), 7.23-7.30 (m, 2H, H-3' and H-5'), 7.34 (dd, 1H, 3J = 9.6 Hz, 4J = 2.1 Hz, H-5), 7.39 (ddd, 1H, 3J = 8.4 Hz, 3J = 6.3 Hz, 4J = 1.7 Hz, H-6), 8.75 (dd, 1H, 3J = 8.0 Hz, 4J = 1.5 Hz, H-1), 8.99 (dd, 1H, 3J = 7.9 Hz, 4J = 1.2 Hz, H-8); 1H,1H-COSY (C6D6): cross-peaks: 1.42-1.49/3.58, 1.83-1.90/4.22, 6.74/7.05, 6.74/8.75, 6.91/7.23-7.30, 6.97/7.23-7.30, 7.05/8.75, 7.09/7.39, 7.09/8.99, 7.34/7.39; 1H,1H-NOESY (C6D6): cross-peaks: 1.42-1.49/3.58, 1.83-1.90/7.34, 3.33/6.74, 3.58/6.91, 4.22/7.34, 6.74/7.05, 6.91/7.23-7.30, 6.97/7.25-7.30, 7.05/8.75, 7.09/7.39, 7.09/8.99, 7.34/7.39; 13C NMR (C6D6) δ: 27.37, 27.49, 52.61, 56.59, 67.71, 115.51, 116.34, 117.11, 121.00, 121.70, 122.21, 122.70, 124.71, 127.37, 128.67, 130.44, 133.98, 135.88, 145.98, 150.67, 160.15, 178.47; 13C,1H-COSY (C6D6): cross-peaks: 27.37/1.83-1.90, 27.49/1.42-1.49, 52.61/4.22, 56.59/3.33, 67.71/3.58, 115.51/6.91, 116.34/6.74, 117.11/7.34, 121.00/8.75, 121.70/6.97, 122.21/7.09, 122.70/7.05, 128.67/8.99, 130.44/7.23-7.30, 133.98/7.39; 13C,1H-COLOC (C6D6): cross-peaks: 52.61/4.22 (1J), 56.59/3.33 (1J), 67.71/3.58 (1J), 115.51/6.97 (3J), 116.34/8.75 (3J), 117.11/7.09 (3J), 121.70/6.91 (3J), 122.21/7.34 (3J), 122.70/6.74 (2J), 124.71/7.09 (3J), 124.71/7.34 (3J), 127.37/7.05 (3J), 130.44/7.23-7.30 (1J), 133.98/7.09 (2J), 133.98/7.39 (1J), 133.98/8.99 (3J), 135.88/6.74 (3J), 135.88/8.75 (3J), 145.98/7.39 (3J), 145.98/8.99 (3J), 150.67/3.33 (3J), 150.67/7.05 (3J), 160.15/7.23-7.30 (3J), 178.47/8.75 (3J), 178.47/8.99 (3J); MS (EI) m/z 373 [M+] (69), 280 (3), 238 (100), 223 (59), 210 (7), 166 (6), 149 (54), 107 (46), 77 (23), 65 (7), 55 (14); Anal. calcd for C24H23NO3: C, 77.19; H, 6.21; N, 3.75; found: C, 77.09; H, 6.38; N, 3.72.
5.3.16. Kokusagenine and skimmianine derivatives
5.3.16.1. Extraction of kokusagenine (29) and skimmianine (30) from Ruta graveolens
The isolation was carried out according to Ohta et al. [21]. Fine cut, dried herb Ruta graveolens (6 kg) were extracted twice with 20 L of methanol for 48 h. The solvent was filtered off and evaporated. The black syrupy residue was refluxed with 10 L of HCl (2.5 M) for 3–4 h and stirred afterwards at room temperature for 15 min. After filtration, the aqueous phase was extracted with diethylether, alkalized with concentrated ammonia and extracted again with CH2Cl2. The solvent was evaporated and the resulting oily residue was purified by MPLC. In a first step we used Al2O3 (90 active, basic, 63–200 µm) as column material and a mixture of toluene and acetone (7:1) as solvent. For the second step the residue was purified over a silica gel column (40–63 µm) with a mixture of toluene and methanol (100:1) to give 404 mg of 29 and 1.5 g of 30. Identity was confirmed by TLC with authentic samples.
5.3.16.2. 4-Hydroxy-6,7-dimethoxyfuro[2,3-b]quinoline (29a)
In a slight modification of the methods of Lamberton et al. [29] and Ishii et al. [30] 4,6,7-trimethoxyfuro[2,3-b]quinoline (29, kokusagenine, 800 mg, 3.1 mmol) was dissolved in 70 mL ethanol and 14 mL of HCl (10 M) and refluxed for 10 h. After cooling the precipitate was filtered off and 25 ml NaOH (3 M) were added. The remaining insoluble residue was removed and the solution was acidified with glacial acetic acid to give 29a (214 mg, 28%) as a light grayish powder: Mp 235 °C dec; 1H NMR (DMSO-d6) δ: 3.89 (s, 3H, 6-OCH3 or 7-OCH3), 3.91 (s, 3H, 6-OCH3 or 7-OCH3), 5.97 (s, 1H, 4-OH), 7.42 (s, 2H, H-3 und H-6), 7.56 (s, 1H, H-5), 7.78 (s, 1H, H-2); 13C NMR (DMSO-d6) δ: 55.26, 55.25, 102.80, 103.23, 105.25, 105.61, 115.16, 138.88, 141.61, 145.35, 151.51, 162.48, 162.72, 172.23; MS (EI) m/z 245 [M+] (100), 230 (26), 202 (23), 187 (8), 174 (8), 159 (10), 122 (5), 50 (5); HRMS (C13H11NO4) calcd. 245.0688, found 245.0680. Without further purification 29a was used for the synthesis of compound 34.
5.3.16.3. 6,7-Dimethoxy-4-(4-phenoxybutoxy)furo[2,3-b]quinoline (34)
4-Hydroxy-6,7-dimethoxyfuro[2,3-b]quinoline (29a, 178 mg, 0.73 mmol), 4-PBB (371 mg, 1.62 mmol) and K2CO3 (477 mg, 3.45 mmol) in 20 mL of dried DMF (120 °C) were reacted according to general procedure B. The crude product was purified by MPLC (cyclohexane/ethyl acetate/methanol (30:9:1)) and afterwards recrystallized from ethanol to give 34 (108 mg, 38%) as a light yellow solid: Mp 130 °C; 1H NMR (DMSO-d6) δ: 1.97-2.10 (m, 4H, 4-O-CH2-(CH2)2-CH2-O-C6H5), 3.84 (s, 3H, 6-OCH3 or 7-OCH3), 3.92 (s, 3H, 6-OCH3 or 7-OCH3), 4.10 (t, 2H, 3J = 6.0 Hz, 4-O-(CH2)3-CH2-O-C6H5), 4.76 (t, 2H, 3J = 5.9 Hz, 4-O-CH2-(CH2)3-O-C6H5), 6.88-6.93 (m, 3H, H-2', H-4' and H-6'), 7.19-7.29 (m, 3H, H-8, H-3', H-5'), 7.32 (d, 1H, 3J = 2.8 Hz, H-3), 7.44 (s, 1H, H-5), 7.92 (d, 1H, 3J = 2.7 Hz, H-2); 13C NMR (DMSO-d6) δ: 25.30, 25.95, 55.30, 55.54, 66.91, 71.09, 100.00, 102.33, 105.14, 106.58, 112.44, 114.35, 120.34, 129.33, 141.90, 143.08, 147.49, 152.32, 154.22, 158.45, 162.46; MS (EI) m/z 393 [M+] (18), 258 (3), 245 (9), 230 (6), 149 (100), 107 (98), 95 (5), 79 (10), 77 (23), 55 (31); HRMS calcd for C23H24NO5 [M+H]+ 394.1655, found 394.1642.
5.3.17. 7,8-Dimethoxy-4-(4-phenoxybutoxy)furo[2,3-b]quinoline (35)
In a slight modification of the methods of Lamberton et al. [29] and Ishii et al. [30] 4,7,8-trimethoxyfuro[2,3-b]quinoline (30, skimmianine, 323 mg, 1.25 mmol) was heated under reflux in 13 mL of ethanol and 2.6 mL HCl (10 M) for 10 h. After cooling the precipitate was filtered off and 25 mL of NaOH (3 M) were added. In an additional filtration the insoluble residue was removed and the filtrate was acidified by using glacial acetic acid to give 4-hydroxy-7,8-dimethoxyfuro[2,3-b]quinoline (30a, yield: 70%). Without any further purification or characterization 30a (245 mg, 1 mmol), 4-PBB (599 mg, 2.61 mmol) and K2CO3 (361 mg, 2.61 mmol) were heated in 35 mL of dried DMF (120 °C) in the presence of a catalytic amount of KI according to procedure B. The crude product was purified by MPLC (cyclohexane/ethyl acetate (3:2)) and recrystallized from ethanol to give 35 (52 mg, 11%) as a white solid: Mp 107.5 °C; 1H NMR (DMSO-d6) δ: 1.98-2.09 (m, 4H, 4-O-CH2-(CH2)2-CH2-O-C6H5), 3.92 (s, 3H, 7-OCH3 or 8-OCH3), 3.95 (s, 3H, 7-OCH3 or 8-OCH3), 4.09 (t, 2H, 3J = 5.9 Hz, 4-O-(CH2)3-CH2-O-C6H5), 4.78 (t, 2H, 3J = 5.7 Hz, 4-O-CH2-(CH2)3-O-C6H5), 6.90-6.94 (m, 3H, H-2', H-4' and H-6'), 7.24-7.41 (m, 4H, H-3, H-6, H-3' and H-5'), 7.95-7.98 (m, 2H, H-2 and H-5); 13C NMR (DMSO-d6) δ: 25.28, 25.86, 56.51, 60.67, 66.85, 71.22, 101.99, 105.31, 112.54, 114.30, 114.36, 117.73, 120.32, 129.33, 140.66, 141.38, 151.79, 155.91, 158.45, 163.68; MS (EI) m/z 393 [M+] (12), 224 (4), 230 (7), 149 (84), 107 (100), 94 (6), 79 (11), 77 (25), 55 (36), 44 (7), 41 (17); HRMS calcd for C23H24NO5 [M+H]+ 394.1655, found 394.1653.
5.3.18. 7-Methyl-4-(4-phenoxybutoxy)-5H-furo[3,2-g][1]benzopyran-5-one (36)
According to Schonberg et al. [31] 4-methoxy-7-methyl-5H-furo[3,2-g][1]benzopyran-5-one (30, visnagin, 2.5 g, 10.9 mmol) was dissolved in HCl (5.5 M) and the solution was refluxed for 1 h. After cooling the precipitate was filtered off and recrystallized from methanol/water to give 4-hydroxy-7-methyl-5H-furo[3,2-g][1]benzopyran-5-one (31a, 1.7 g, 72%). The spectroscopic data were in accordance with literature [32]. In a second step 31a (432 mg, 2 mmol), CsF-celite (1.9 g, 9 mmol) and 4-PBB (1.39 g, 6.1 mmol), dissolved in 5 mL of dried acetonitrile, were reacted according to general procedure A. The crude product was purified by MPLC (cyclohexane/ethyl acetate (3:1)) and recrystallized from methanol/water to give 34 (575 mg, 79%) as a white solid: Mp 90 °C; 1H NMR (DMSO-d6) δ: 1.91-1.95 (m, 4H, 4-O-CH2-(CH2)2-CH2-O-C6H5), 2.31 (s, 3H, 7-CH3), 4.04 (t, 2H, 3J = 5.8 Hz, 4-O-(CH2)3-CH2-O-C6H5), 4.29 (t, 2H, 3J = 5.6 Hz, 4-O-CH2-(CH2)3-O-C6H5), 6.02 (s, 1H, H-6), 6.88-6.93 (m, 3H, H-2', H-4' and H-6'), 7.17 (d, 1H, 3J = 2.2 Hz, H-3), 7.23-7.29 (m, 2H, H-3' and H-5'), 7.49 (s, 1H, H-9), 8.03 (d, 1H, 3J = 2.3 Hz, H-2); 13C NMR (DMSO-d6) δ: 19.30, 25.31, 26.35, 67.09, 73.86, 95.11, 105.29, 110.15, 112.12, 114.35, 117.26, 120.28, 129.37, 146.46, 151.92, 155.29, 156.90, 158.58, 164.01, 176.47; MS (EI) m/z 364 [M+] (69), 271 (57), 229 (78), 216 (87), 201 (33), 149 (62), 107 (100), 77 (64), 55 (70), 43 (39); Anal. calcd for C22H20O5: C, 72.51; H, 5.53; found: C, 72.61; H, 5.71.
5.3.19. 9-Methoxy-7-methyl-4-(4-phenoxybutoxy)-5H-furo[3,2-g][1]benzopyran-5-one (37)
4,9-Dimethoxy-7-methyl-5H-furo[3,2-g][1]benzopyran-5-one (32, khellin, 1.35 g, 5.19 mmol) was dissolved in 15 mL of CH2Cl2 and demethylated with boron tribromide (482 µL, 5 mmol) according to procedure C. The crude compound was purified by MPLC (cyclohexane/CH2Cl2/methanol (5:5:1)) to give 4-hydroxy-9-methoxy-7-methyl-5H-furo[3,2-g][1]benzopyran-5-one (32a, yield: 60%). The spectroscopic data were in accordance with literature [23]. In a second step 32a (456 mg, 1.85 mmol), CsF-celite (1.81 g, 8.5 mmol) and 4-PBB (895 mg, 3.9 mmol), dissolved in 5 mL of dried acetonitrile, were reacted according to general procedure A. The crude product was purified by MPLC (cyclohexane/ethyl acetate (3:1)) to give 37 (191 mg, 26%) as a white solid: Mp 105 °C; 1H NMR (CDCl3) δ: 2.05-2.09 (m, 4H, 4-O-CH2-(CH2)2-CH2-O-C6H5), 2.39 (s, 3H, 7-CH3), 4.08 (t, 2H, 3J = 5.9 Hz, 4-O-(CH2)3-CH2-O-C6H5), 4.20 (s, 3H, 9-OCH3), 4.23 (t, 2H, 3J = 6.0 Hz, 4-O-CH2-(CH2)3-O-C6H5), 6.03 (s, 1H, H-6), 6.89-6.94 (m, 3H, H-2', H-4' and H-6'), 6.97 (d, 1H, 3J = 2.3 Hz, H-3), 7.24-7.29 (m, 2H, H-3' and H-5'), 7.60 (d, 1H, 3J = 2.2 Hz, H-2); 1H,1H-NOESY (CDCl3): cross-peaks: 2.05-2.09/6.97, 2.29/6.03, 4.23/6.97, 6.79/7.60; 13C NMR (DMSO-d6) δ: 19.43, 25.34, 26.34, 61.13, 67.08, 74.31, 105.15, 109.93, 113.33, 114.33, 119.36, 120.27, 129.35, 145.67, 146.50, 146.93, 148.08, 158.57, 164.11, 176.57; MS (EI) m/z 394 [M+] (12), 259 (37), 246 (13), 231 (100), 149 (83), 107 (85), 94 (13), 79 (14), 77 (39), 55 (29); Anal. calcd for C23H22O6: C, 70.04; H, 5.62; found: C, 70.20; H, 5.80.
5.3.20. 8-(4-Phenoxybutoxy)psoralen (38)
8-Methoxypsoralen (33, 8-MOP, 720 mg, 3.33 mmol) was demethylated with boron tribromide (337 µL, 3.5 mmol) according to procedure C. The crude compound was recrystallized from methanol/water to give 8-hydroxypsoralen (33a, yield: 90%). Without any further characterization 33a (520 mg, 2.57 mmol), 4-PBB (2.36 g, 10.3 mmol) and K2CO3 (1.03 g, 7.46 mmol) in 25 mL of dried acetone were reacted in a second step according to general procedure B. The crude product was purified by MPLC (cyclohexane/ethyl acetate (4:1)) to give 38 (486 mg, 54%) as a slightly yellow solid: Mp 53 °C; 1H NMR (DMSO-d6) δ: 1.92-2.08 (m, 4H, 8-O-CH2-(CH2)2-CH2-O-C6H5), 4.05 (t, 2H, 3J = 6.1 Hz, 8-O-(CH2)3-CH2-O-C6H5), 4.46 (t, 2H, 3J = 6.1 Hz, 8-O-CH2-(CH2)3-O-C6H5), 6.42 (d, 1H, 3J = 9.5 Hz, H-3), 6.87-6.93 (m, 3H, H-2", H-4" and H-6"), 7.08 (d, 1H, 3J = 2.2 Hz, H-4'), 7.22-7.29 (m, 2H, H-3" and H-5"), 7.66 (s, 1H, H-5), 8.09 (d, 1H, 3J = 2.2 Hz, H-5'), 8.12 (d, 1H, 3J = 9.7 Hz, H-4); 13C NMR (DMSO-d6) δ: 25.19, 26.26, 66.91, 73.09, 106.97, 113.95, 114.08, 114.34, 116.36, 120.28, 125.68, 129.32, 130.84, 142.81, 145.13, 147.32, 147.67, 158.52, 159.65; MS (EI) m/z 350 [M+] (2), 202 (16), 174 (10), 149 (100), 107 (87), 94 (8), 89 (13), 77 (34), 63 (9), 55 (39), 51 (8); Anal. calcd for C21H18O5: C, 71.99; H, 5.18; found: C, 71.65; H, 5.30.
5.3.21. 5-(4-Phenoxybutoxy)-2H-[1]benzopyran-2-one (42)
According to Dubuffet et al. [33] 2,6-dimethoxybenzaldehyde (40, 5 g, 30.09 mmol) and methoxycarbonylmethylene-triphenylphosphorane (12.1 g, 36.1 mmol) were refluxed in 90 ml toluene. After completion of the reaction (controlled by TLC) the solvent was evaporated and 90 mL diethylether was added. The triphenylphosphinoxide was filtered off and the solvent was evaporated. The crude product was purified by MPLC (cyclohexane/ethyl acetate (4:1)) to give 3-(2,6-dimethoxyphenyl)acryl acid methylester (40, yield: 72%). For identification 1H NMR data were recorded: 1H NMR (DMSO-d6) δ: 3.71 (s, 3H, -COO-CH3), 3.87 (s, 6H, 2'-OCH3 and 6'-OCH3), 6.73 (d, 2H, 3J = 8.5 Hz, H-3' and H-5'), 6.78 (d, 1H, 3J = 16.3 Hz, H-α), 7.37 (t, 1H, 3J = 8.4 Hz, H-4'), 7.99 (d, 1H, 3J = 16.3 Hz, H-β). In a second step 40 (4.8 g, 21.6 mmol) was demethylated with boron tribromide (4.18 mL, 43.4 mmol) according to procedure C. The crude compound was purified by MPLC (cyclohexane/ethyl acetate (80:20)) to give 5-hydroxy-2H-[1]benzopyran-2-one (41, yield: 65%). 1H NMR data were in accordance to literature [34]. In a final step 41 (520 mg, 3.2 mmol), CsF-celite (2.97 g, 14 mmol) and 4-PBB (1.47 g, 6.4 mmol), dissolved in 5 mL of dried acetonitrile, were reacted according to general procedure A. The crude product was purified by MPLC (cyclohexane/ethyl acetate (3:1)) and recrystallized from methanol to give 42 (546 mg, 55%) as a white solid: Mp 82 °C; 1H NMR (DMSO-d6) δ: 1.87-1.98 (m, 4H, 5-O-CH2-(CH2)2-CH2-O-C6H5), 4.05 (t, 2H, 3J = 5.9 Hz, 5-O-(CH2)3-CH2-O-C6H5), 4.19 (t, 2H, 3J = 5.8 Hz, 5-O-CH2-(CH2)3-O-C6H5), 6.35 (d, 1H, 3J = 9.7 Hz, H-3), 6.89-6.95 (m, 5H, H-6, H-8, H-2', H-4' and H-6'), 7.24-7.30 (m, 2H, H-3' and H-5'), 7.53 (t, 1H, 3J = 8.4 Hz, H-7), 8.08 (d, 1H, 3J = 9.7 Hz, H-4); 13C NMR (DMSO-d6) δ: 25.20, 25.64, 51.42, 66.71, 108.68, 114.35, 116.50, 120.39, 123.09, 125.77, 126.73, 129.36, 131.90, 139.52, 144.38, 158.42, 176.18; MS (EI) m/z 310 [M+] (8), 217 (7), 175 (8), 163 (6), 149 (73), 107 (100), 94 (10), 79 (17), 77 (40), 55 (52); Anal. calcd for C19H18O4: C, 73.53; H, 5.85; found: C, 73.58; H, 6.00.
5.3.22. 4-(4-Phenoxybutoxy)benzofuran (45)
According to Kneen et al. [35] 6,7-dihydro-5H-benzofuran-4-one (43, 5 g, 36.7 mmol), 10 mL of 1-dodecene and 3.3 g palladium-charcoal (10% Pd) in 60 mL decalin were heated at reflux under nitrogen atmosphere for 20 h. After the reaction mixture was cooled down to 80 °C 50 mL of ethanol were added. The resulting precipitate was filtered off and purified by MPLC (cyclohexane/ethyl acetate (4:1)) to give 4-hydroxybenzofuran (44, 2.22 g, 45%). The spectroscopic data were in accordance with literature [36]. In a second step 44 (2.1 g, 15.7 mmol), 4-PBB (7.21 g, 31.5 mmol) and K2CO3 (6.48 g, 47 mmol) in 25 mL of dried DMF were reacted according to general procedure B. The crude product was purified by MPLC (cyclohexane) to give 45 (300 mg, 7%) as a light yellow oily residue; 1H NMR (DMSO-d6) δ: 2.02-2.15 (m, 4H, 4-O-CH2-(CH2)2-CH2-O-C6H5), 4.12 (t, 2H, 3J = 6.4 Hz, 4-O-(CH2)3-CH2-O-C6H5), 4.20 (t, 2H, 3J = 6.1 Hz, 4-O-CH2-(CH2)3-O-C6H5), 6.70 (dd, 1H, 3J = 7.7 Hz, 5J = 0.5 Hz, H-5 or H-7), 6.89 (dd, 1H, 3J = 2.2 Hz, 5J = 0.7 Hz, H-3), 6.92-7.00 (m, 3H, H-2', H-4' and H-6'), 7.15-7.35 (m, 4H, H-5 or H-7, H-6, H-3', H-5'), 7.56 (d, 1H, 3J = 2.2 Hz, H-2); 13C NMR (DMSO-d6) δ: 25.39, 66.93, 67.59, 103.80, 104.16, 104.57, 114.37, 117.16, 120.33, 125.11, 129.36, 144.36, 152.46, 155.55, 158.53; MS (EI) m/z 282 [M+] (12), 149 (88), 134 (23), 107 (100), 94 (9), 77 (46), 65 (8), 55 (54), 51 (17), 41 (7); Anal. calcd for C18H18O3: C, 76.57; H, 6.43; found: C, 76.42; H, 6.73.
5.3.23. 4-(4-Phenoxybutoxy)-5H-furo[3,2-g][1]benzopyran-5-one (51)
In a first step according to Spath et al. [37] 4-methoxy-7-methyl-5H-furo[3,2-g][1]benzopyran-5-one (46, visnagin, 5 g, 21.7 mmol) was refluxed for 15 min in 500 mL of KOH (0.2 M). After cooling 75 mL H2SO4 (0.4 M) were added and the precipitate was filtered off. The precipitate was recrystallized from methanol/water (60:40) to give 5-acetyl-4-methoxybenzofuran-6-ole (47, visnaginone, yield: 83%). For identification 1H NMR data were recorded: 1H NMR (DMSO-d6) δ: 2.54 (s, 3H, 5-COCH3), 4.12 (s, 3H, 4-OCH3), 6.71 (s, 1H, H-7), 7.18 (d, 1H, 3J = 2 Hz, H-3), 7.82 (d, 1H, 3J = 3 Hz, H-2), 11.77 (s, 1H, 6-OH). In a next step according to Abdel-Rahman et al.[38] 47 (4.12 g, 20 mmol) and N,N-dimethylformamide dimethylacetal (DMFDMA) were refluxed in 50 mL of toluene for 3 h. The solvent was evaporated and the resulting residue was washed with petroleum ether and recrystallized from ethanol to give 1-(4-methoxy-6-hydroxy-benzofuran-5-yl)-3-dimethylamino-2-propen-1-one (48, 65%). Without further characterization 48 (3.38 g, 12.9 mmol) was refluxed in glacial acetic acid for 5 h. After adding ice the solution was alkalized with a solution of NH4OH (7 M). The forming precipitate was filtered off and recrystallized from ethanol to give 4-methoxy-5H-furo[3,2-g][1]benzopyran-5-one (49, yield: 80%). The spectroscopic data were in accordance with literature [38]. In a next step 49 (865 mg, 4 mmol) was demethylated with boron tribromide (385 µL, 4 mmol) according to procedure C. The crude compound was recrystallized from methanol/water (5:1) to give 4-hydroxy-5H-furo[3,2-g][1]benzopyran-5-one (50, 98%). For characterization 1H NMR and 13C NMR data were recorded: 1H NMR (DMSO-d6) δ: 6.38 (d, 1H, 3J = 5.9 Hz, H-7), 7.09 (dd, 1H, 3J = 2.3 Hz, 5J = 0.9 Hz, H-3), 7.37 (d, 1H, 5J = 0.9 Hz, H-9), 8.03 (d, 1H, 3J = 2.3 Hz, H-2), 8.34 (d, 1H, 3J = 5.9 Hz, H-6), 13.56 (s, 1H, 4-OH); 13C NMR (DMSO-d6) δ: 91.08, 103.86, 106.24, 109.60, 112.39, 146.39, 153.73, 154.25, 158.19, 158.49, 183.28. In a final step 50 (800 mg, 3.96 mmol), CsF-celite (5.72 g, 27 mmol) and 4-PBB (1.83 g, 8 mmol), dissolved in 5 mL of dried acetonitrile, were reacted according to general procedure A. The crude product was purified by MPLC (cyclohexane/ethyl acetate (7:3)) to give 51 (554 mg, 40%) as a light yellow solid: Mp 70 °C; 1H NMR (DMSO-d6) δ: 1.93-1.99 (m, 4H, 4-O-CH2-(CH2)2-CH2-O-C6H5), 4.05 (t, 2H, 3J = 5.8 Hz, 4-O-(CH2)3-CH2-O-C6H5), 4.31 (t, 2H, 3J = 5.7 Hz, 4-O-CH2-(CH2)3-O-C6H5), 6.13 (d, 1H, 3J = 5.9 Hz, H-7), 6.88-6.93 (m, 3H, H-2', H-4' and H-6'), 7.19 (d, 1H, 3J = 1.5 Hz, H-3), 7.23-7.29 (m, 2H, H-3' and H-5'), 7.54 (s, 1H, H-9), 8.04 (d, 1H, 3J = 2.2 Hz, H-2), 8.10 (d, 1H, 3J = 5.9 Hz, H-6); 13C NMR (DMSO-d6) δ: 25.26, 26.32, 67.08, 73.85, 95.29, 105.27, 112.48, 113.38, 114.34, 117.30, 120.24, 129.31, 146.53, 152.04, 154.40, 155.18, 156.98, 158.56, 175.94; MS (EI) m/z 350 [M+] (5), 257 (79), 215 (54), 202 (100), 149 (83), 107 (99), 79 (24), 77 (68), 55 (65), 51 (24); Anal. calcd for C21H18O5: C, 71.99; H, 5.18; found: C, 72.18; H, 5.34.
5.3.24. 5-Hydroxy-2-methyl-6,7-methylendioxy-4H-[1]benzopyran-4-one (55)
The oxidation of 46 (10 g, 43.5 mmol) was carried out according to Schonberg et al. [39] in 300 mL of H2SO4 (1 M) at 60 °C. To this solution 20 mL of a potassium dichromate solution (0.3 M) was added dropwise so that the temperature remained between 60–70 °C. Immediately a white precipitate formed, which was filtered off after stirring the reaction for an additional 2 h, washed and recrystallized from methanol/water to give 6-formyl-7-hydroxy-5-methoxy-2-methyl-4H-[1]benzopyran-4-one (52, 71%). Without any further characterization 52 was used for the next step. According to Gammill et al. [40] 52 (4.7 g, 20.1 mmol) was dissolved in 34 mL of NaOH (1 M) and on cooling 12 mL of a H2O2 solution (10 M) were added dropwise. The reaction mixture was stirred for 2–3 h at room temperature, acidified with HCl (3 M) and extracted of ethyl acetate (3 × 50 mL). The pooled ethyl acetate phases were dried over Na2SO4 and the solvent was evaporated to give 6,7-dihydroxy-5-methoxy-2-methyl-4H-[1]benzopyran-4-one (53, 86%). For identification 1H NMR data were recorded: 1H NMR (DMSO-d6) δ: 2.24 (s, 3H, 2-CH3), 3.71 (s, 3H, 5-OCH3), 5.90 (s, 1H, H-8), 6.62 (s, 1H, H-3), 9.47 (s, 2H, 6-OH and 7-OH). According to Gammill et al. [40] 53 (3.84 g, 17.3 mmol) was then dissolved in 86.5 mL DMF and K2CO3 (5.19 g, 34.6 mmol) was added. A solution of dibromomethane (3.31 g, 19.03 mmol) in DMF (63 mL) was added dropwise under nitrogen atmosphere. Afterwards the reaction mixture was stirred at 95 °C for 4 h. K2CO3 was removed and solvent was evaporated. The remaining residue was dissolved in diethylether and extracted three times with NaOH (0.25 M). The diethylether was evaporated and the residue was purified by MPLC (cyclohexan/ethyl acetate (3:2)) to give 2-methyl-6,7-methylendioxy-5-methoxy-4H-[1]-benzopyran-4one (54, 35.4%). For identification 1H NMR data were recorded: 1H NMR (DMSO-d6) δ: 2.26 (s, 3H, 2-CH3), 3.87 (s, 3H, 5-OCH3), 5.98 (s, 1H, H-8), 6.16 (s, 2H, -O-CH2-O-), 6.90 (s, 1H, H-3). In a next step 54 (850 mg, 3.63 mmol) was demethylated with boron tribromide (347 µL, 3.6 mmol) according to procedure C. The crude compound was purified by MPLC (cyclohexane/ethyl acetate (1:1)) to give 5-hydroxy-2-methyl-6,7-methylendioxy-4H-[1]benzopyran-5-one (55, 85%) as a yellowish powder: Mp 174 °C; 1H NMR (DMSO-d6) δ: 2.37 (s, 3H, 2-CH3), 6.15 (s, 2H, H-2'), 6.23 (s, 1H, H-8), 6.78 (s, 1H, H-3), 12.84 (s, 1H, 5-OH). 13C NMR (DMSO-d6) δ: 19.75, 89.41, 102.64, 106.27, 107.86, 129.29, 141.02, 152.99, 153.65, 167.99, 182.39; MS (EI) m/z 220 [M+] (100), 191 (10), 162 (54), 134 (29), 122 (8), 119 (8), 78 (28), 69 (22), 66 (22), 53 (29), 43 (23); HRMS calcd for C11H9O5 [M+H]+ 221.0450, found 221.0444.
5.3.25. 2-Methyl-6,7-methylendioxy-5-(4-phenoxybutoxy)-4H-[1]benzopyran-4-one (56)
55 (238 mg, 1.08 mmol), 4-PBB (495 mg, 2.16 mmol) and 430 mg (3.12 mmol) K2CO3 in 15 mL of dried acetone were reacted according to general procedure B. The crude product was purified by MPLC (cyclohexane/ethyl acetate (4:1)) to give 56 (80 mg, 20%) as a yellow solid: Mp 83.5 °C; 1H NMR (DMSO-d6) δ: 1.82-2.00 (m, 4H, 5-O-CH2-(CH2)2-CH2-O-C6H5), 2.31 (s, 3H, 2-CH3), 4.07 (t, 2H, 3J = 6.2 Hz, 5-O-(CH2)3-CH2-O-C6H5), 4.20 (t, 2H, 3J = 6.0 Hz, 5-O-CH2-(CH2)3-O-C6H5), 6.01 (s, 1H, H-8), 6.17 (s, 2H, H-2'), 6.92-6.98 (m, 4H, H-3, H-2", H-4" and H-6"), 7.27-7.35 (m, 2H, H-3" and H-5"); 13C NMR (DMSO-d6) δ: 19.05, 25.17, 26.03, 66.98, 72.95, 93.48, 102.33, 110.55, 112.42, 114.34, 120.23, 129.31, 135.77, 139.08, 152.16, 154.16, 158.55, 163.37, 175.53; MS (EI) m/z 368 [M+] (3), 275 (100), 233 (80), 220 (71), 162 (22), 149 (24), 107 (52), 77 (61), 65 (20), 55 (35); Anal. calcd for C21H20O6: C, 68.47; H, 5.47; found: C, 68.73; H, 5.69.
5.3.26. 2,6-Dihydro-8-methyl-5-(4-phenoxybutoxy)pyrano[3,2-g][1]benzopyran-2,6-dione (59)
In a first step 46 was converted into 52 as described for 55. In a second step according to Atta et al. [41] a mixture of 52 (7.2 g, 30.8 mmol) and methoxycarbonylmethylenetriphenylphosphorane (10.3 g, 30.8 mmol) in 500 mL of toluene was refluxed for 6 h. After evaporation of the solvent under vacuum the remaining residue was purified by MPLC (CH2Cl2/ethyl acetate (10:1)) to give 2,6-dihydro-5-methoxy-8-methylpyrano[3,2-g][1]benzopyran-2,6-dione (57, 25%). The structure was confirmed by 1H NMR: 2.34 (s, 3H, 8-CH3), 3.90 (s, 3H, 5-OCH3), 6.15 (d, 1H, 4J = 0.8 Hz, H-7), 6.49 (d, 1H, 3J = 9.8 Hz, H-3), 7.35 (d, 1H, 5J = 0.5 Hz, H-10), 8.15 (dd, 1H, 3J = 9.8 Hz, 5J = 0.5 Hz, H-4). In a next step 57 (290 mg, 1.12 mmol) was demethylated with boron tribromide (139 µL, 1.44 mmol) according to procedure C to give 2,6-dihydro-5-hydroxy-8-methylpyrano[3,2-g][1]benzopyran-2,6-dione (58, 91%). For characterization 1H NMR data were recorded: 1H NMR (DMSO-d6) δ: 2.44 (s, 3H, 8-CH3), 6.38-6.41 (m, 2H, H-3 and H-7), 7.04 (s, 1H, H-10), 8.09 (d, 1H, 3J = 9.6 Hz, H-4), 13.94 (s, 1H, 5-OH). Without further purification 58 (180 mg, 0.74 mmol), 4-PBB (336.8 mg, 1.47 mmol) and K2CO3 (306.4 mg, 2.22 mmol) in 30 mL of dried DMF (120 °C) were reacted according to general procedure B. The crude product was purified by MPLC (dichlormethane/ethyl acetate (200:1)) to give 59 (27 mg, 9%) as a white crystals: Mp 120-124 °C; 1H NMR (DMSO-d6) δ: 1.90-2.01 (m, 4H, 5-O-CH2-(CH2)2-CH2-O-C6H5), 2.35 (s, 3H, 8-CH3), 4.06 (t, 2H, 3J = 6.1 Hz, 5-O-(CH2)3-CH2-O-C6H5), 4.10 (t, 2H, 3J = 6.3 Hz, 5-O-CH2-(CH2)3-O-C6H5), 6.15 (s, 1H, H-7), 6.48 (d, 1H, 3J = 9.8 Hz, H-3), 6.89-6.94 (m, 3H, H-2', H-4' and H-6'), 7.24-7.30 (m, 2H, H-3' and H-5'), 7.36 (s, 1H, H-10), 8.14 (d, 1H, 3J = 9.8 Hz, H-4); 13C NMR (DMSO-d6) δ: 19.27, 25.23, 26.16, 67.07, 76.29, 100.81, 110.94, 111.24, 113.95, 114.37, 115.31, 120.31, 129.35, 138.67, 156.09, 158.53, 158.86, 158.94, 164.91, 175.19; MS (EI) m/z 392 [M+] (15), 299 (42), 257 (85), 244 (43), 229 (34), 149 (53), 107 (95), 79 (33), 77 (83), 55 (100); HRMS calcd for C23H20O6 [M]+ 392.1260, found 392.1252.
5.3.27. 5-Methoxy-4-methyl-8-(4-phenoxybutoxy)psoralen (63)
According to Atta et al. [41] 5-acetyl-4,7-dimethoxybenzofuran-6-ole (60, 1.36 g, 5.76 mmol) and methoxycarbonylmethylenetriphenylphosphorane (1.93 g, 5.76 mmol) were refluxed in 96 mL dried toluene for 3 h. After cooling to room temperature the precipitate was filtered off and recrystallized from toluene to give 5,8-dimethoxy-4-methylpsoralen (61, yield: 85%). The spectroscopic data were in accordance with literature [41]. In a next step 61 (520 mg, 2 mmol) was demethylated with boron tribromide (96 µL, 1 mmol) according to procedure C. The crude compound was purified by MPLC (toluene/methanol (50:1)) to give 8-hydroxy-5-methoxy-4-methylpsoralen (62, 43%). Without any further characterization 62 (370 mg, 1.5 mmol), CsF-celite (1.39 g, 6.75 mmol) and 4-PBB (688 mg, 3 mmol) were reacted in a final step according to general procedure A to give 5-methoxy-4-methyl-8-(4-phenoxybutoxy)psoralen (63, 149 mg, 25%) as a white solid: Mp 76 °C; 1H NMR (CDCl3) δ: 2.03-2.11 (m, 4H, 8-O-CH2-(CH2)2-CH2-O-C6H5), 2.63 (s, 3H, 4-CH3), 4.06 (s, 3H, 5-OCH3), 4.09 (t, 2H, 3J = 6.0 Hz, 8-O-(CH2)3-CH2-O-C6H5), 4.40 (t, 2H, 3J = 6.1 Hz, 8-O-CH2-(CH2)3-O-C6H5), 6.11 (s, 1H, H-3), 6.89-6.92 (m, 3H, H-2", H-4" and H-6"), 6.94 (d, 1H, 3J = 2.3 Hz, H-4'), 7.23-7.29 (m, 2H, H-3" and H-5"), 7.59 (d, 1H, 3J = 2.3 Hz, H-5'); 1H,1H-NOESY (CDCl3): cross-peaks: 2.63/4.06, 2.63/6.11, 4.06/6.94, 6.94/7.59; 13C NMR (DMSO-d6) δ: 23.26, 25.22, 26.20, 61.27, 66.91, 73.09, 105.47, 108.72, 112.97, 114.34, 116.59, 120.28, 127.02, 129.34, 143.58, 146.19, 148.67, 154.09, 158.53, 158.95; MS (EI) m/z 394 [M+] (5), 246 (9), 231 (21), 203 (7), 149 (100), 107 (69), 79 (10), 77 (23), 65 (6), 55 (23); Anal. calcd for C23H22O6: C, 70.04; H, 5.62; found: C, 70.25; H, 5.80.
5.3.28. 4-(4-Phenoxybutoxy)furo[2,3-b]quinoline (71)
According to Morel et al. [42] iodomethane (4.47 mL, 71.8 mmol) was added to a premixed solution of 2,4-dihydroquinoline (64, 2.98 g, 17.95 mmol) and silver carbonate (15 g, 53.85 mmol) in 50 mL of anhydrous hexane. The greenish solution was stirred for 3 days at room temperature in the dark. Afterwards ethyl acetate was added in order to dilute the sluggish mixture. The grey solution was filtrated through celite and concentrated under vacuum. The crude residue was purified by flash chromatography (pentane/ethyl acetate (19:1)) to give 2,4-dimethoxyquinoline (65, montanine, 1.36 g, 40%) as white needles. According to Bhoga et al. [43] n-butyllithium (6.72 mL, 10.75 mmol, 1.6 M solution in hexane) was added dropwise at −10 °C to a solution of 65 (1.13 g, 5.97 mmol) in 15 mL of anhydrous diethylether. The orange solution was maintained at this temperature for 45 min and 0.69 mL of anhydrous DMF (8.96 mmol) in 3 mL of diethylether was added. The reaction mixture was stirred at 0 °C for 30 min and at room temperature for another 45 min. During this time, the red solution turned light pink. The mixture was subsequently hydrolyzed (20 mL of water; color turned from pink to yellow) and extracted with diethylether (3 × 20 mL). The combined organic extracts were washed with brine, dried over MgSO4 and finally concentrated under vacuum. The crude residue was purified by flash chromatography (pentane/ethyl acetate (19:1)) to give 2,4-dimethoxyquinoline-3-carbaldehyde (66, 699.4 mg, 54%) as a white solid. In a next step 9.47 mL of a solution of potassium tert-butoxide in THF (1 M) was added to methoxymethyltriphenylphosphonium chloride (3.35 g, 9.47 mmol) in 29 mL of anhydrous diethylether. This homogeneous mixture was stirred at room temperature for 1 h. 66 (1.03 g, 4.74 mmol) in 13 mL of dry THF was then added and the resulting red solution was stirred over night. Afterwards the organic solvents were evaporated and the crude residue was directly purified by flash chromatography (pentane/ethyl acetate (9:1)) to give 2,4-dimethoxy-3-(2-methoxyethyenyl)quinoline (67, 1.14 g, 98%) as a 6:1 (E/Z) mixture as a white solid. The spectroscopic data were in accordance with literature [44]. In a next step 67 (1.9 g, 7.75 mmol) was treated with 30 mL of HCl (5.5 M), refluxed for 45 min and then carefully quenched with NaHCO3. The extraction of the neutral solution with diethylether (3 × 30 mL) gave 2-hydroxy-4-methoxy-3-quinolineacetaldehyde (68) as a crude orange-brown residue (~50%), which was treated without any further purification with 30 g of polyphosphoric acid. The resulting sluggish mixture was heated at 125 °C for 5 h, poured into water, neutralized carefully with NaHCO3 and extracted with chloroform. The resulting residue was subsequently purified by flash chromatography (pentane/ethyl acetate (7:3)) to give 4-methoxyfuro[2,3-b]quinoline (69, dictamnine, 418.4 mg, 27% overall yield for the 2 steps) as a white powder. The melting point (131 °C; Lit. 132 °C [45]) and the spectroscopic data were in accordance to literature [43]. In a next step 0.22 mL of isopropylthiol were added to a solution of sodium hydride (258 mg, 6.4 mmol) in 4 mL of anhydrous DMF and stirred for 10 min. Afterwards a solution of 69 (183.4 mg, 0.92 mmol) in 4 mL DMF was added dropwise. This reaction mixture was heated under reflux for 4 h, cooled down to room temperature, neutralized at 0 °C with HCl (2 M) and extracted with diethylether. The combined organic extracts were subsequently washed with brine, dried over Na2SO4 and concentrated under vacuum. The crude product was purified by flash chromatography (pentane/ethyl acetate (5:5)) to give nordictamnine (70, 40.8 mg, 24%) as a white solid. In a last step KHCO3 (152 mg, 1.10 mmol) and 4-PBB (78 mg, 0.34 mmol) were added to a solution of 70 (40.8 mg, 0.22 mmol) in 3 mL of 2-butanone. The mixture was heated under reflux for 25 h, cooled down to 5 °C (ice bath) and carefully quenched with HCl (1 M). The aqueous solution was extracted with diethylether and the combined organic extracts were subsequently washed with brine, dried over Na2SO4 and concentrated under vacuum. The crude product was purified by flash chromatography (pentane/ethyl acetate (4:6)) to give 71 (20.1 mg, 27%): Mp 101-102 °C; 1H NMR (500 Hz, CDCl3) δ: 2.14 (m, 2H) and 2.21 (m, 2H) (4-O-CH2-(CH2)2-CH2-O-C6H5), 4.13 (t, 2H, 3J = 6.0 Hz, 4-O-(CH2)3-CH2-O-C6H5), 4.79 (td, 2H, 3J = 6.0 Hz, 4J = 1.6 Hz, 4-O-CH2-(CH2)3-O-C6H5), 6.93 (t, 2H, 3J = 7.8 Hz, H-2' and H-6'), 6.97 (t, 1H, 3J = 7.4 Hz, H-4'), 7.03 (m, 1H, H-3), 7.30 (m, 2H, H-3' and H-5'), 7.45 (t, 1H, 3J = 7.4 Hz, H-6), 7.63 (d, 1H, 3J = 3 Hz, H-2), 7.70 (td, 1H, 3J = 7.4 Hz, 4J = 1.6 Hz, H-7), 8.03 (d, 1H, 3J = 8.5 Hz, H-5), 8.29 (d, 1H, 3J = 8.0 Hz, H-8); MS (FAB) m/z 334 [M+H]+ (75), 333 [M+] (15), 186 (37), 154 (45), 149 (100), 136 (55); HRMS calcd for C21H20NO3 [M+H]+ 334.1445, found 334.1443.
5.3.29. 9-(4-Phenoxybutyl)furo[2,3-b]quinolin-4(9H)-one (72)
For synthesis see compound 71. The crude product was purified by flash chromatography (pentane/ethyl acetate (4:6)) to give 72 (yield: 5%): Mp 105 °C; 1H NMR (500 Hz, CDCl3) δ: 1.95 (quint, 2H, 3J =7.5 Hz , N-(CH2)2-CH2-CH2-O-C6H5), 2.14 (quint, 2H, 3J = 6.0 Hz, N-CH2-CH2-(CH2)2-O-C6H5), 4.06 (t, 2H, 3J = 6 Hz, N-(CH2)3-CH2-O-C6H5), 4.56 (t, 2H, 3J = 6 Hz, N-CH2-(CH2)3-O-C6H5), 6.91 (d, 2H, 3J = 7.8 Hz, H-2' and H-6'), 6.89 (t, 1H, 3J = 7.5 Hz, H-4'), 7.16 (swide, 1H, H-3), 7.32-7.27 (m, 3H, H-2, H-3' and H-5'), 7.41 (t, 1H, 3J = 7.5 Hz, H-6), 7.57 (d, 1H, 3J = 8.3 Hz, H-8), 7.70 (t, 1H, 3J = 7 Hz, H-7), 8.62 (d, 1H, 3J = 8 Hz, H-5); HRMS calcd for C21H20NO3 [M+H]+ 334.1443, found 334.1439.
5.3.30. 4-(4-Phenoxybutoxy)-7H-furo[3,2-g]chromene-7-thione (73)
PAP-1 (2, 500 mg, 1.43 mmol) was dissolved in 20 mL of anhydrous toluene. The resulting solution was refluxed and Lawesson’s reagent (3.86 g, 9.24 mmol) was added at the start of the reaction and again after 10 h. After 20 h the solution was concentrated in vacuo. Purification of the crude product by flash chromatography (pentane/ethyl acetate (7:3)) gave 73 (36 mg, 15%) as an orange powder: Mp 126-127 °C; 1H NMR (500 MHz, CDCl3) δ: 2.04 (m, 2H, 4-O-(CH2)2-CH2-CH2-O-C6H5), 2.11 (m, 2H, 4-O-CH2-CH2-(CH2)2-O-C6H5), 4.08 (t, 2H, 3J = 5.8 Hz, 2H, 4-O-(CH2)3-CH2-O-C6H5), 4.57 (t, 2H, 3J = 6.0 Hz, 4-O-CH2-(CH2)3-O-C6H5), 6.89 (d, 2H, 3J = 7.1 Hz, H-2' and H-6'), 6.98 (m, 1H, H-3), 7.08 (d, 1H, 3J = 9.5 Hz, H-6), 7.30-7.26 (m, 4H, H-9, H-3', H-4', H-5'), 7.62 (d, 1H, 3J = 3.0 Hz, H-2), 7.88 (d, 1H, 3J = 9 Hz, H-5); 13C NMR (CDCl3) δ: 25.9, 26.9, 67.0, 72.5, 93.7, 105.2, 108.5, 113.9, 114.4, 120.8, 125.6, 129.5, 130.7, 145.4, 148.9, 154.9, 158.4, 158.7, 183.2, 198.1; MS (ES+) m/z 367 [M+H]+ (8), 366 (40), 218 (12), 174 (20), 149 (90), 107 (100); HRMS calcd for C21H18O4S [M+] 366.0926, found 366.0921.
5.4. Electrophysiology
The inhibition of Kv1.3 was determined by whole-cell patch-clamp on L929 cells stably expressing Kv1.3 with a holding potential of −80 mV [17]. The currents were recorded in normal Ringer solution (160 mM NaCl, 4.5 mM KCl, 2 mM CaCl2, 1 mM MgCl2, 10 mM HEPES, pH 7.4, 290–310 mOsm) with an internal pipette solution containing 145 mM KF, 2 mM MgCl2, 10 mM HEPES, 10 mM EGTA (pH 7.2, 290–310 mOsm). Depolarizing pulses to 40 mV were applied every 45 s for 500 ms. Kv1.5 currents were recorded from MEL-C88 cells. IC50 values were determined by fitting the reduction of area under the current curve to the Hill equation.
Acknowledgements
The authors thank Mr. Björn Henke for excellent technical assistance. This research was funded by R21NS53426 from NIH (to H.W.).
Abbreviations
- TCM
central memory T cell
- TEM
effector memory T cell
- 5-MOP
5-methoxypsoralen
- MPLC
middle pressure liquid chromatography
- PAP
phenoxyalkoxypsoralen
- 4-PBB
4-phenoxybutyl bromide
- DMFDMA
N,N-dimethylformamide dimethylacetal
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Chandy KG, Wulff H, Beeton C, Pennington M, Gutman GA, Cahalan MD. Potassium channels as targets for specific immunomodulation. Trends Pharmacol. Sci. 2004;25:280–289. doi: 10.1016/j.tips.2004.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Wulff H, Calabresi PA, Allie R, Yun S, Pennington M, Beeton C, Chandy KG. The voltage-gated Kv1.3 K+ channel in effector memory T cells as new target for MS. J. Clin. Invest. 2003;111:1703–1713. doi: 10.1172/JCI16921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Beeton C, Wulff H, Standifer NE, Azam P, Mullen KM, Pennington MW, Kolski-Andreaco A, Wei E, Grino A, Counts DR, Wang PH, Leehealey CJ, B SA, Sankaranarayanan A, Homerick D, Roeck WW, Tehranzadeh J, Stanhope KL, Zimin P, Havel PJ, Griffey S, Knaus HG, Nepom GT, Gutman GA, Calabresi PA, Chandy KG. Kv1.3 channels are a therapeutic target for T cell-mediated autoimmune diseases. Proc. Natl. Acad. Sci. U S A. 2006;103:17414–17419. doi: 10.1073/pnas.0605136103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Rus H, Pardo CA, Hu L, Darrah E, Cudrici C, Niculescu T, Niculescu F, Mullen KM, Allie R, Guo L, Wulff H, Beeton C, Judge SI, Kerr DA, Knaus HG, Chandy KG, Calabresi PA. The voltage-gated potassium channel Kv1.3 is highly expressed on inflammatory infiltrates in multiple sclerosis brain. Proc. Natl. Acad. Sci. U S A. 2005;102:11094–11099. doi: 10.1073/pnas.0501770102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Koo GC, Blake JT, Talento A, Nguyen M, Lin S, Sirotina A, Shah K, Mulvany K, Hora D, Jr, Cunningham P, Wunderler DL, McManus OB, Slaughter R, Bugianesi R, Felix J, Garcia M, Williamson J, Kaczorowski G, Sigal NH, Springer MS, Feeney W. Blockade of the voltage-gated potassium channel Kv1.3 inhibits immune responses in vivo. J. Immunol. 1997;158:5120–5128. [PubMed] [Google Scholar]
- 6.Beeton C, Pennington MW, Wulff H, Singh S, Nugent D, Crossley G, Khaytin I, Calabresi PA, Chen CY, Gutman GA, Chandy KG. Targeting effector memory T cells with a selective peptide inhibitor of Kv1.3 channels for therapy of autoimmune diseases. Mol. Pharmacol. 2005;67:1369–1381. doi: 10.1124/mol.104.008193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Beeton C, Barbaria J, Giraud P, Devaux J, Benoliel A, Gola M, Sabatier J, Bernard D, Crest M, Beraud E. Selective blocking of voltage-gated K+ channels improves experimental autoimmune encephalomyelitis and inhibits T cell activation. J. Immunol. 2001;166:936–944. doi: 10.4049/jimmunol.166.2.936. [DOI] [PubMed] [Google Scholar]
- 8.Beeton C, Wulff H, Barbaria J, Clot-Faybesse O, Pennington M, Bernard D, Cahalan MD, Chandy KG, Beraud E. Selective blockade of T lymphocyte K+ channels ameliorates experimental autoimmune encephalomyelitis, a model for multiple sclerosis. Proc. Natl. Acad. Sci. USA. 2001;98:13942–13947. doi: 10.1073/pnas.241497298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Valverde P, Kawai T, Taubman MA. Selective blockade of voltage-gated potassium channels reduces inflammatory bone resorption in experimental periodontal disease. J. Bone Miner. Res. 2004;19:155–164. doi: 10.1359/JBMR.0301213. [DOI] [PubMed] [Google Scholar]
- 10.Chandy KG, Wulff H, Beeton C, Calabresi PA, Gutman GA, Pennington MW. Kv1.3 Potassium Channel: Physiology, Pharmacology and Therapeutic Indications. In: Triggle DJ, Gopalakrishnan M, Rampe D, Zheng W, editors. Voltage-gated ion channels as drug targets. Weinheim: Wiley-VCH; 2006. pp. 214–274. [Google Scholar]
- 11.Wulff H, Beeton C, Chandy KG. Potassium channels as therapeutic targets for autoimmune disorders. Curr. Opin. Drug Discovery Dev. 2003;6:640–647. [PubMed] [Google Scholar]
- 12.Bohuslavizki KH, Koppenhofer E, Hansel W, Moller WD. A new approach for the treatment of demyelinating diseases? J. Neuroimmunol. 1988;20:251–252. doi: 10.1016/0165-5728(88)90170-1. [DOI] [PubMed] [Google Scholar]
- 13.Bohuslavizki KH, Hansel W, Kneip A, Koppenhofer E, Niemoller E, Sanmann K. Mode of action of psoralens, benzofurans, acridones and coumarins on the ionic currents in intact myelinated nerve fibres and its significance in demyelinating diseases. Gen. Physiol. Biophys. 1994;13:309–328. [PubMed] [Google Scholar]
- 14.Wulff H, Rauer H, During T, Hanselmann C, Ruff K, Wrisch A, Grissmer S, Haensel W. Alkoxypsoralens, novel nonpeptide blockers of Shaker-type K+ channels: synthesis and photoreactivity. J. Med. Chem. 1998;41:4542–4549. doi: 10.1021/jm981032o. [DOI] [PubMed] [Google Scholar]
- 15.Bohuslavizki HK, Hinck-Kneip C, Kneip A, Koppenhofer E, Reimers A. Reduction of MS-related scotoma by a new class of potassium channel blockers from Ruta graveolens. Neuroopthalmol. 1993;13:191–198. [Google Scholar]
- 16.Vennekamp J, Wulff H, Beeton C, Calabresi PA, Grissmer S, Hansel W, Chandy KG. Kv1.3 blocking 5-phenylalkoxypsoralens: a new class of immunomodulators. Mol. Pharmacol. 2004;65:1364–1373. doi: 10.1124/mol.65.6.1364. [DOI] [PubMed] [Google Scholar]
- 17.Schmitz A, Sankaranarayanan A, Azam P, Schmidt-Lassen K, Homerick D, Hansel W, Wulff H. Design of PAP-1, a selective small molecule Kv1.3 blocker, for the suppression of effector memory T cells in autoimmune diseases. Mol. Pharmacol. 2005;68:1254–1270. doi: 10.1124/mol.105.015669. [DOI] [PubMed] [Google Scholar]
- 18.Pereira LE, Villinger F, Wulff H, Sankaranarayanan A, Raman G, Ansari AA. Pharmacokinetics, toxicity, and functional studies of the selective Kv1.3 channel blocker 5-(4-phenoxybutoxy)psoralen in rhesus macaques. Exp. Biol. Med. (Maywood) 2007;232:1338–1354. doi: 10.3181/0705-RM-148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Azam P, Sankaranarayanan A, Homerick D, Griffey S, Wulff H. Targeting effector memory T cells with the small molecule Kv1.3 blocker PAP-1 suppresses allergic contact dermatitis. J. Invest. Dermatol. 2007;127:1419–1429. doi: 10.1038/sj.jid.5700717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Wulff H, Pennington M. Targeting effector memory T-cells with Kv1.3 blockers. Curr. Opin. Drug Discovery Dev. 2007;10:438–445. [PubMed] [Google Scholar]
- 21.Ohta T, Mori Y, Noda C, Aoki T. Furoquinolines. XX. Alkaloids of the leaves of Ruta graveolens and synthesis of dihydrokokusaginine. Chem. Pharm. Bull. 1960;8:377–379. [Google Scholar]
- 22.Butenschon I, Moller K, Hansel W. Angular methoxy-subsituted furo- and pyranoquinolinones as blockers of the voltage-gated potassium channel Kv1.3. J. Med. Chem. 2001;44:1249–1256. doi: 10.1021/jm001007u. [DOI] [PubMed] [Google Scholar]
- 23.Harvey AJ, Baell JB, Toovey N, Homerick D, Wulff H. A new class of blockers of the voltage-gated potassium channel Kv1.3 via modification of the 4- or 7-position of khellinone. J. Med. Chem. 2006;49:1433–1441. doi: 10.1021/jm050839v. [DOI] [PubMed] [Google Scholar]
- 24.Baell JB, Gable RW, Harvey AJ, Toovey N, Herzog T, Hansel W, Wulff H. Khellinone derivatives as blockers of the voltage-gated potassium channel Kv1.3: synthesis and immunosuppressive activity. J. Med. Chem. 2004;47:2326–2336. doi: 10.1021/jm030523s. [DOI] [PubMed] [Google Scholar]
- 25.Schmalhofer WA, Bao J, McManus OB, Green B, Matyskiela M, Wunderler D, Bugianesi RM, Felix JP, Hanner M, Linde-Arias A-R, Ponte CG, Velasco L, Koo G, Staruch MJ, Miao S, Parsons WH, Rupprecht K, Slaughter RS, Kaczorowski GJ, Garcia ML. Identification of a new class of inhibitors of the voltage-gated potassium channel, Kv1.3, with immunosuppressant properties. Biochemistry. 2002;18:7781–7794. doi: 10.1021/bi025722c. [DOI] [PubMed] [Google Scholar]
- 26.Felix JP, Bugianesi RM, Schmalhofer WA, Borris R, Goetz MA, Hensens OD, Bao JM, Kayser F, Parsons WH, Rupprecht K, Garcia ML, Kaczorowski GJ, Slaughter RS. Identification and biochemical characterization of a novel nortriterpene inhibitor of the human lymphocyte voltage-gated potassium channel, Kv1.3. Biochemistry. 1999;38:4922–4930. doi: 10.1021/bi982954w. [DOI] [PubMed] [Google Scholar]
- 27.Lee JC, Choi Y. An improved method for preparation of carboxylic esters using CsF-celite/alkyl halide/CH3CN combination. Synth. Commun. 1998;28:2021–2026. [Google Scholar]
- 28.Yamamoto K, Jikei M, Miyatake K, Katoh J, Nishide H, Tsuchida E. Sulfide bond formation for the synthesis of poly(thioarylene) through oxidation of sulfur chloride with aromatics. Macromolecules. 1994;27:4312–4317. [Google Scholar]
- 29.Lamberton JA, Price JR. Alkaloids of the Australian Rutaceae: Acronychia baueri. IV. Alkaloids present in the leaves. Aust. J. Chem. 1953;6:66–77. [Google Scholar]
- 30.Ishii H. Alkaloids of Rutaceous plants. XIV. Syntheses of some hydrogenated derivatives of skimmianine. Yakugaku Zasshi. 1961;81:248–253. [Google Scholar]
- 31.Schonberg A, Badran N. Khellin from visnagin. J. Am. Chem. Soc. 1951;73:2960–2961. [Google Scholar]
- 32.Ahluwalia VK, Arora KK, Prakash C. Selective Claisen migration in chromones. A convenient synthesis of the linear furanochromones desmethoxyvisnagin, norvisnagin, and visnagin. Gazz. Chim. Ital. 1981;111:103–107. [Google Scholar]
- 33.Dubuffet T, Loutz A, Lavielle G. An efficient large scale synthesis of coumarins by a dealkylative boron-mediated ring closure of 3-(ortho-methoxyaryl)propenoic esters. Synth. Commun. 1999;29:929–936. [Google Scholar]
- 34.Cerqueira NMFSA, Oliveira-Campos AMF, Coelho PJ, Melo De Carvalho LH, Samat A, Guglielmetti R. Synthesis of photochromic dyes based on annulated coumarin systems. Helv. Chim. Acta. 2002;85:442–450. [Google Scholar]
- 35.Kneen G, Maddocks PJ. A convenient synthesis of 4-hydroxybenzo(b)furan, (karanjol) Synth. Commun. 1986;16:1635–1640. [Google Scholar]
- 36.Bonini C, Cristiani G, Funicello M, Viggiani L. Facile entry to 4- and 5-hydroxybenzofuran and to their amino derivatives. Synth. Commun. 2006;36:1983–1990. [Google Scholar]
- 37.Spath E, Gruber W. Natural chromones. II. Constitution of visnagin (from Ammi visnaga) Ber. Dtsch. Chem. Ges. 1941;74B:1492–1500. [Google Scholar]
- 38.Abdel-Rahman A-RH, Keshk EM, El-Telbani EM. Linearly fused furochromones by intramolecular enaminone reactions. Z. Naturforsch. B: Chem. Sci. 2002;57:557–562. [Google Scholar]
- 39.Schonberg A, Aziz G. Furochromones and Coumarins. VI. Demethylation of xantotoxin, khellin and khellol with aniline hydrochloride and magnesium iodide. Am. J. Chem. Soc. 1953;75:3265–3266. [Google Scholar]
- 40.Gammill RB, Nash SA. Oxymetallation of khellin. Solvomercuration, osmylation, and palladium-catalyzed oxidation of the furan ring in khellin. The synthesis of highly oxygenated chromones and 2-substituted furochromones. J. Org. Chem. 1986;51:3116–3123. [Google Scholar]
- 41.Atta SMS, Hafez TS, Mahran MR. Organophosphorus chemistry. 25. The utilization of Wittig reagents in lactone ring formation. Application to the synthesis of linear furocoumarins and pyranocoumarins. Phosphorus Sulfur and Silicon Relat. Elem. 1993;80:109–116. [Google Scholar]
- 42.Morel AF, Larghi EL, Selvero MM. Mild, efficient and selective silver carbonate-mediated O-alkylation of 4-hydroxy-2-quinolones. Synthesis of 2,4-dialkoxyquinolines. Synlett. 2005:2755–2758. [Google Scholar]
- 43.Bhoga U, Mali RS, Adapa SR. New synthesis of linear furoquinoline alkaloids. Tetrahedron Lett. 2004;45:9483–9485. [Google Scholar]
- 44.Narasimhan NS, Mali RS. Synthetic application of lithiation reactions. VI. New synthesis of linear furoquinoline alkaloids. Tetrahedron. 1974;30:4153–4157. [Google Scholar]
- 45.Grundon MF, McCorkindale NJ. Synthesis of g-fagarine and dictamnine. Chem. Ind. (London, United Kingdom) 1956:1091–1092. [Google Scholar]