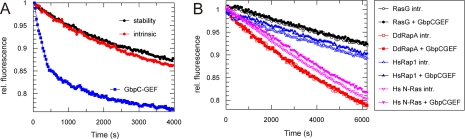
FIGURE 1.
The Roc domain of GbpC is activated by the GbpC-RasGEF domain. A, the purified Roc-Cor fragment of GbpC was loaded with the fluorescent GDP analog mGDP, and the decay of fluorescence was followed over time in the presence of excess GDP. The stability of the protein was measured by incubating the mGDP-loaded protein in assay buffer without further additions. The intrinsic exchange activity was measured by incubating the mGDP-loaded protein with an excess of unlabeled GDP. The addition of the purified RasGEF domain of GbpC causes a dramatic increase in the exchange rate, meaning that GbpC uses its own RasGEF to activate the Roc domain. B, same assay as in A, but the Roc-Cor fragment of GbpC was replaced by several other GTPases. Intrinsic exchange rates and exchange rates in the presence of GbpC-RasGEF are shown. No differences were found upon the addition of the GbpC-RasGEF domain, confirming specificity of GbpC-RasGEF for its own Roc. All of the exchange assays were repeated at least twice on different days, yielding similar results. Dd, Dictyostelium discoideum; Hs, Homo sapiens.