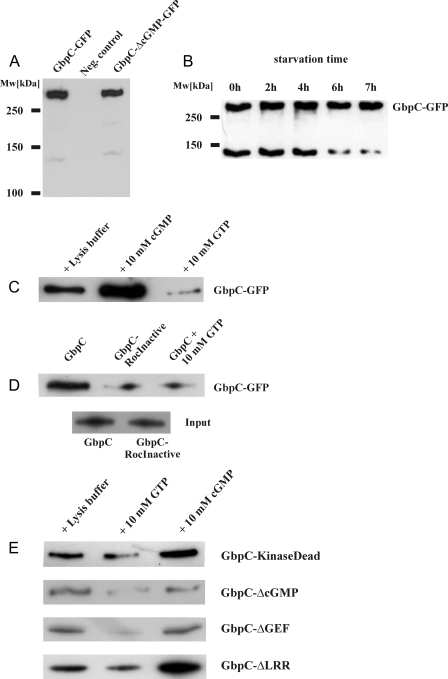
FIGURE 3.
Roc-mediated GTP-binding by GbpC is specific and is stimulated by cGMP. A and B, full-length GbpC-GFP (∼320 kDa) and GbpC-ΔcGMP-GFP, expressed in gbpC null cells, can be visualized by Western blotting. A truncated protein (∼140 kDa) is also visible, and its presence is developmentally regulated. Equal amounts of cells were taken at several time points during starvation. The cells were boiled in SDS loading buffer and resolved by SDS-PAGE, and GbpC-GFP expression was visualized with Western blotting, using anti-GFP antibodies. C, GbpC-GFP was expressed in gbpC null cells and pulled down from lysates with GTP-agarose beads in the presence of equal volumes of lysis buffer, cGMP, or GTP, respectively. Bound protein was visualized on a Western blot, using anti-GFP antibodies. D, GbpC and the GbpC-RocInactive mutant with an inactive Roc domain were pulled down with GTP-agarose. The results show very little binding of GbpC-RocInactive to GTP-agarose. The inputs are shown as a control, indicating equal expression levels of both constructs. The proteins were visualized on a Western blot, using anti-GFP antibodies. E, cGMP stimulates GTP binding in mutants with abolished kinase or LRR domains but does not stimulate GTP binding in a mutant that is unable to bind cGMP or in a mutant with a deletion of the catalytic part of the RasGEF domain. GFP-fused GbpC mutants were pulled down with GTP-agarose as in A and visualized with anti-GFP antibodies on a Western blot. Representatives of at least three independent experiments on different days are presented.