FIGURE 1.
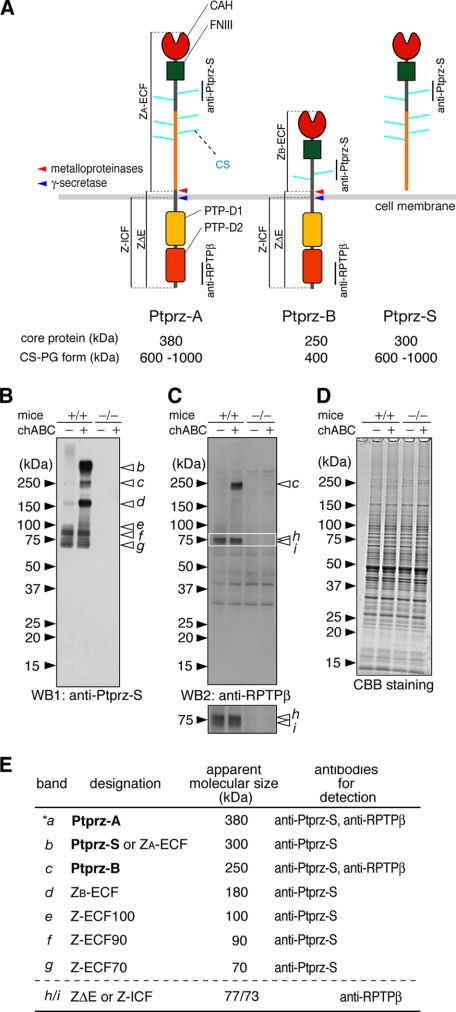
Novel protein species of Ptprz in the adult mouse brain. A, schematic representation of Ptprz isoforms. Molecular sizes of the chondroitin sulfate proteoglycan forms (CS-PG) and their core proteins after treatment with chABC are shown (3). Regions corresponding to the epitopes of antibodies used in this study are indicated by vertical lines. We designated the proteolytic fragments as follows: ZA-ECF or ZB-ECF, the extracellular fragment of Ptprz-A or Ptprz-B; ZΔE, the membrane-tethered fragment of Ptprz-A and Ptprz-B; Z-ICF, the intracellular fragment cleaved from ZΔE. The cleavage sites are indicated by arrows (red, metalloproteinases including TACE and MMP-9; blue, presenilin/γ-secretase). Domains are highlighted in different colors: CAH, carbonic anhydrase-like domain; FNIII, fibronectin type III domain; PTP-D1 and PTP-D2, tyrosine phosphatase domains. B–D, brain extract (5 μg of protein) of wild-type mice (+/+) and Ptprz-deficient mice (-/-) was treated with (+) or without (-) chABC. The samples were separated on a 5–20% gradient gel followed by Western blotting (WB) using anti-Ptprz-S (B). The same blot was stripped and reprobed with anti-RPTPβ (C). The lower image is a vertical enlargement of the area enclosed by a rectangle in the upper image. Staining with Coomassie Brilliant Blue R-250 to check the amounts of protein applied (D). The figures are representative of five separate experiments. E, summary of the immunoreactive bands (b–i). Their designation, apparent molecular size, and specific antibodies for detection are shown. The core protein of Ptprz-A (band *a) was scarcely detected in the brain extract by Western blotting, but it was evident in immunoprecipitation assays (data not shown).