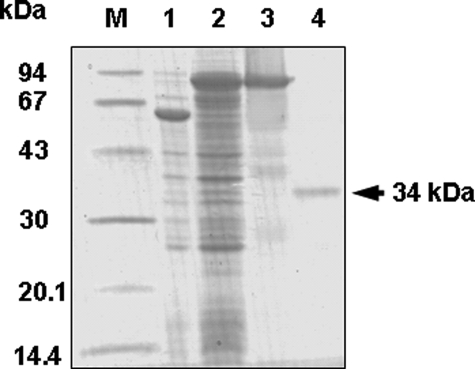
FIGURE 3.
Production of recombinant Euglena ALase. The soluble proteins were extracted from E. coli cells transformed with an empty vector pColdTF or the vector containing the Euglena ALase (pColdTF/EgALase) after isopropyl 1-thio-β-d-galactopyranoside induction. The recombinant His- and TF-tag protein was purified in a nickel-nitrilotriacetic acid column. HRV3C protease was used to detach the fused tag. The samples were separated by 12.5% SDS-PAGE and visualized with Coomassie Brilliant Blue. M, molecular marker; lane 1, soluble fraction of cell extract transformed with an empty pColdTF vector; lane 2, soluble fraction of cell extract transformed with pColdTF/EgALase; lane 3, elution fraction from nickel-nitrilotriacetic acid; lane 4, purified recombinant EgALase after treatment with HRV 3C protease.