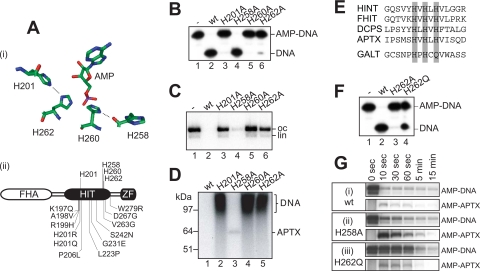
FIGURE 5.
The four conserved active site histidine residues are critical for DNA deadenylation by APTX. A, panel i, arrangement of the histidine triad residues His-258, His-260, His-262, and His-201 in the active site of APTX based on crystallographic analysis of human HINT1, a related HIT hydrolase, with bound AMP (19). Hydrogen bonds between His-201 and His-262 and between His-258 and His-260 are indicated. His-260 is the nucleophilic residue that forms the phosphohistidine bond with the substrate. Panel ii, primary structure of APTX indicating the four histidines and the amino acid changes found in AOA1 patients. B, DNA deadenylation by wild-type (wt) and active site mutants of APTX (10 nm) determined using the oligonucleotide-based SSB substrate shown in Fig. 1B. Deadenylation was determined by denaturing PAGE followed by autoradiography. C, DNA deadenylation by wild-type and active site mutants of APTX (50 nm) determined using the adenylated DNA substrate of Fig. 4A. After 5 min at 37 °C, remaining 32P-labeled DNA adenylates were visualized by agarose gel electrophoresis followed by autoradiography. oc, open circle; lin, linear. D, aliquots from reactions shown in C were analyzed by SDS-PAGE/autoradiography to detect 32P-labeled adenylated DNA and 32P-labeled APTX. E, sequence alignment of the indicated HIT superfamily proteins from humans. The hydrolases feature histidines at all three position of the HIT motif (gray shading), whereas the transferase galactose-1-phosphate uridylyltransferase (GALT) is characterized by a glutamine in the third position of the motif. FHIT, fragile histidine triad protein; DCPS, decapping enzyme, scavenger. F, a transferase-like version of APTX with glutamine at His-262 (APTXH262Q; 10 nm) was analyzed for deadenylation activity in comparison with APTX and APTXH262A as described in B. G, time course of the deadenylation activity and covalent AMP-APTX intermediate formation of wild-type APTX (panel i) and the APTXH258A (panel ii) and APTXH262Q (panel iii) mutants as determined in C and D.