FIGURE 2.
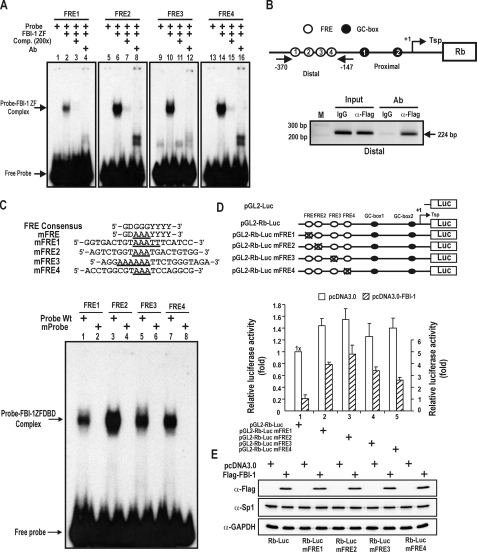
FBI-1 binds to the distal FRE clusters of the endogenous Rb promoter. Characterization of the functional role of the FRE elements in transcription. A, EMSA. Four 32P-labeled FRE1–4 oligonucleotide probes (bp –308 to –300, –298 to –290, –244 to –236, and –188 to –180) were incubated with recombinant FBI-1ZFDBD (aa 382–490) and separated on a 4% non-denaturating polyacrylamide gel. B, ChIP assays of FBI-1 binding at the FRE cluster on the endogenous Rb gene. Structure of the Rb gene promoter and locations of PCR primer sets used for ChIP assays. pcDNA3-FLAG-FBI-1 expression plasmid was transfected into HeLa cells, and chromatin was immunoprecipitated with anti-FLAG antibody or control IgG. Arrows, primers used in ChIP; Tsp (+1), transcription start site. C, EMSA. Sequence comparisons of the FRE consensus sequence and mutant FRE probes. The core GGG sequence of the FREs was substituted with AAA in mutant probes. EMSA was carried out as in A. FBI-1ZFDBD was able to bind to the wild type, but not to any of the mutant probes. D, functional characterization of FBI-1 binding to FRE elements by transient transcription analysis in HeLa cells. Also shown are the structures of the pGL2-Rb-Luc Wt and mutant plasmids tested. Open circles, FBI-1 binding sites; black circles, Sp1-binding GC-boxes. X, mutation introduced. Mutations of the FREs increased transcription of Rb gene by 20–130% compared with the Wt promoter. E, Western blot analysis of Sp1 and FBI-1 in the HeLa cells transfected and described in D.