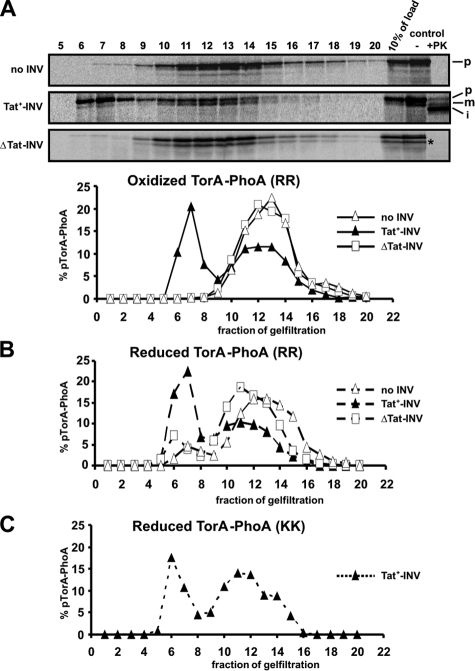
FIGURE 2.
TatABC-dependent membrane association of translocation-competent and translocation-incompetent forms of TorA-PhoA. A, TorA-PhoA was synthesized in vitro under oxidizing conditions in the absence or presence of INV and reactions were stopped by 0.8 mm puromycin. Translocation was assessed as depicted in the control lanes. After removing aggregated material by centrifugation at 16,000 × g for 20 min at 4 °C, 100-μl reaction volumes were applied to a 1.5 ml Sepharose CL-6B column and eluted in 20 × 100 μl fractions, which together with 10% of the load were analyzed by SDS-PAGE. INV uniquely eluted in fractions 6–8 as confirmed by the presence of PK-resistant material (not shown). The radioactivity of each fraction was quantitated using Image Quant 5.2 software (Molecular Dynamics) and the means of 2–5 parallel experiments were plotted. The diagram illustrates the peak of membrane-associated TorA-PhoA only in those assays that contained Tat+-INV. B, same as in A for TorA-PhoA now synthesized under reducing conditions. Despite the fact that reduced TorA-PhoA is not competent for translocation it associated with Tat+-INV in much the same manner as the oxidized TorA-PhoA depicted in panel A. C, membrane association of the KK mutant of TorA-PhoA synthesized under reducing conditions. These data were obtained from a single experiment. Even inactivation of the signal sequence by replacing the RR-consensus with a KK pair does not prevent TorA-PhoA from binding to Tat+-INV.