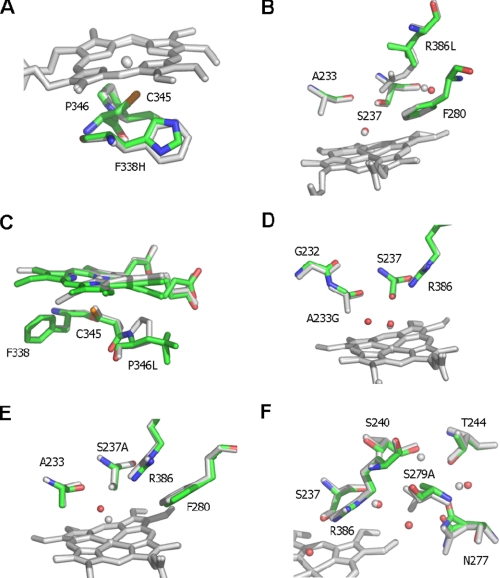
FIGURE 2.
Active site structures of CYP121 mutant enzymes. Atomic structures were determined for each of six CYP121 point mutants in the vicinity of the heme cofactor. The figure shows an overlay of the CYP121 mutant structures with the WT structure (Protein Data Bank code 1N40). Mutant structures are atom-colored with green carbons, whereas the WT structure is in grayscale. With the exception of C, the heme is only shown for the WT structure for clarity. In C (as for other panels) the WT heme is in gray, whereas the heme group of the P346L mutant is shown with green carbons. The P346L heme and Leu346 side chain are observed in two distinct conformations. A more detailed view is presented in Fig. S4 in the supplemental material. Selected side chains are displayed in sticks and associated water molecules in spheres for each overlaid structure. A–F show the overlays of F338H, R386L, P346L, A223G, S237A, and S279A mutants, respectively, with WT CYP121.