FIGURE 6.
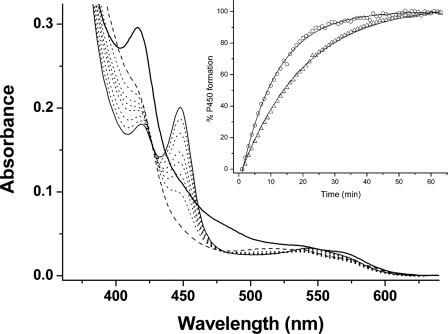
Reconstitution of a Mtb redox system and electron transfer to CYP121. A Mtb redox system of FprA (4 μm), Fdx (18 μm), and WT/mutant CYP121 (4 μm) was set up in CO-saturated buffer (see “Experimental Procedures”). CYP121 complex formation was initiated by NADPH addition (300 μm). Shown is spectral accumulation of the Fe(II)CO form of the F338H CYP121 mutant over time. The initial spectrum (thick line) is prior to NADPH addition and has contributions from oxidized FprA/Fdx proteins. The dashed line spectrum is after NADPH addition and shows bleaching of reductase proteins. Later spectra (dotted lines) were collected at 2, 5, 10, 15, 25, and 35 min. The final spectrum (thin solid line) at 50 min shows a predominantly thiolate-coordinated Fe(II)CO enzyme with Soret at 448 nm. The inset shows a plot of A448 (percentage of P450 formed) versus time, with data fitted using an exponential function for the F338H (open triangles) and R386L (open circles) CYP121 mutants. Rate constants determined for Fe(II)CO complex formation were 0.060 ± 0.001 and 0.089 ± 0.002 min-1, respectively.