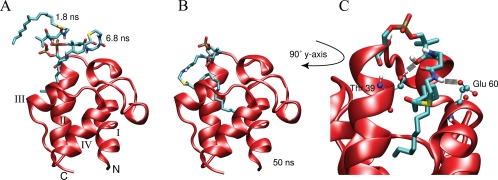
FIGURE 4.
Snapshots of the hexadecanoyl-ACP simulation started with a solvent-exposed acyl chain. The prosthetic group was initially extended away from the protein entirely, extending upwards from helix II. Soon after the start of the simulation, the acyl chain folds onto itself and moves toward the protein as is seen in two snapshots at 1.8 and 6.8 ns (A). When the tip of the acyl chain finds the entrance to the cavity it moves into the pocket, drawing the linker away from transiently formed hydrogen bonds with the protein (B). C, shows a close up view of the prosthetic group inside the hydrophobic cavity along with the two major hydrogen bonding partners, Thr-39 and Glu-60.