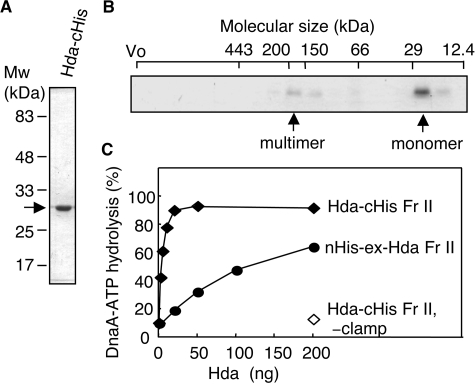
FIGURE 2.
Purification and RIDA activity of Hda-cHis. A, Hda-cHis Fr II (1 μg) was analyzed by SDS-PAGE and Coomassie Brilliant Blue staining. B, Hda-cHis Fr II (55 μg) was analyzed using Superdex-200 PC 3.2/30 gel filtration. Eluted proteins were collected in 30-μl fractions, and portions (0.5 μl) were analyzed by SDS-PAGE and silver staining. The positions of the void volume (Vo) and molecular size markers were determined under the same conditions and are indicated at the top of the gel. Arrows indicate the peak fractions of Hda-cHis. C, activity for DnaA-ATP hydrolysis was assessed using a staged RIDA reconstituted system as described under “Experimental Procedures.” [α-32P]ATP-DnaA (0.5 pmol) was incubated for 20 min at 30 °C in the presence or absence (–clamp) of the DNA-loaded clamp (20 fmol as clamp) in buffer containing 2 mm ATP and the indicated amounts of Hda-cHis Fr II or nHis-ex-Hda Fr II.