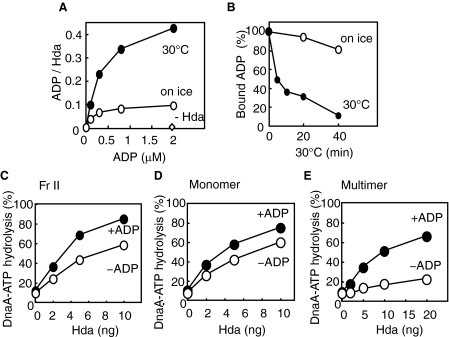
FIGURE 3.
Role for ADP in Hda-cHis activity. A, ADP binding activity of Hda. [3H]ADP was incubated at 30 °C or on ice for 20 min in the presence or absence (–Hda) of Hda-cHis Fr II (1 pmol as a monomer), followed by retention on a nitrocellulose filter. The bound ADP molecules per Hda monomer are presented. B, dissociation of Hda-cHis-bound ADP. Hda-cHis Fr II (18 pmol) was incubated at 30 °C for 20 min in buffer (5 μl) containing 5 μm [3H]ADP. A portion (0.28 μl) of the reaction was diluted in buffer (25 μl) containing 1 μm nonradiolabeled ADP and was further incubated at 30 °C or on ice for the indicated time, followed by the filter-retention analysis. C–E, DnaA-ATP hydrolysis activity was analyzed in a staged RIDA reconstituted system. The DNA-loaded clamps (20 fmol as clamp) were incubated in buffer containing [α-32P]ATP-DnaA (0.5 pmol) in the presence (+ADP) or absence (–ADP) of 30 μm ADP. Hda-cHis monomers and multimers were isolated by gel filtration of Hda-cHis Fr II (see Fig. 2B). C, Hda-cHis Fr II. D, Hda-cHis monomers. E, Hda-cHis multimers.