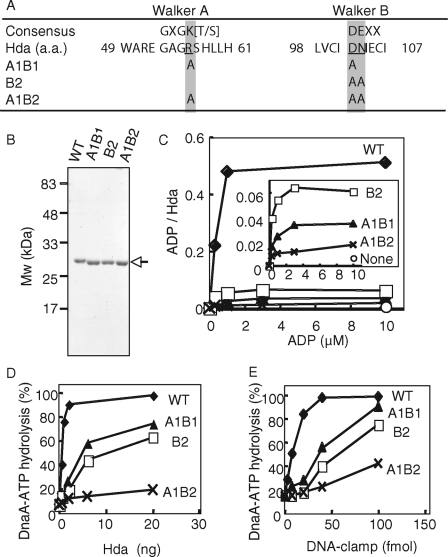
FIGURE 6.
In vitro analysis of Walker motif mutants of Hda. A, amino acid (a.a.) sequence of the Hda Walker A and B motifs and their consensus sequences are shown. The amino acid substitutions in the mutant Hda used in this study are also indicated. B, wild-type (WT) and mutant (A1B1, B2, and A1B2) Hda-cHis proteins (0.5 μg) were purified from insoluble cell lysate fractions (see “Experimental Procedures”) and analyzed by SDS-PAGE and Coomassie Brilliant Blue staining. C, indicated concentrations of [3H]ADP were incubated at 30 °C for 20 min in the presence or absence (None) of wild-type (WT) or mutant (A1B1, B2, or A1B2) Hda-cHis proteins (20 pmol), followed by retention on a nitrocellulose filter. The bound ADP molecules per Hda monomer are presented. The inset shows an expanded view of the data from the mutant Hda-cHis proteins. D and E, DnaA-ATP hydrolysis activity of the wild-type Hda-cHis protein (WT) and the mutant Hda-cHis proteins (A1B1, B2, and A1B2) was assessed in a staged RIDA reconstituted system (30 °C, 20 min) in the presence of 0.5 pmol of [α-32P]ATP-DnaA and 200 μm ADP. D, DNA-loaded clamp (100 fmol as clamp) was included. E, indicated amounts (as clamp) of the DNA-loaded clamp were incubated in the presence of 20 ng of wild-type Hda-cHis protein (WT) or mutant Hda-cHis proteins (A1B1, B2, or A1B2).