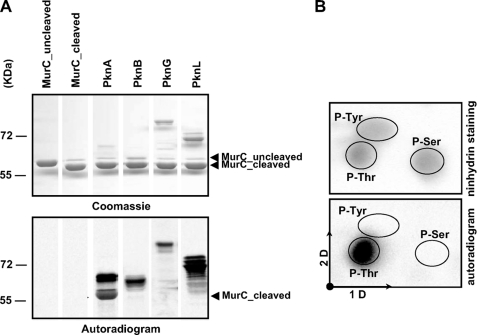
FIGURE 2.
In vitro phosphorylation of C. glutamicum MurC. A, in vitro phosphorylation of MurC by corynebacterial STPKs. The four recombinant STPKs (PknA, PknB, PknG, and PknL) were expressed and purified as previously described by Fiuza et al. (18). Recombinant MurC was treated with the TEV protease to remove the N-terminal His tag and then incubated with [γ-33P]ATP and the different kinases. Samples were separated by SDS-PAGE (upper panel) and visualized by autoradiography (lower panel). Upper bands illustrate the autokinase activity of each STPK, whereas lower bands reflect phosphorylation of MurC. B, phosphoamino acid content of MurC. MurC was phosphorylated in vitro in presence of PknA and [γ-33P]ATP, analyzed by SDS-PAGE, electroblotted onto an Immobilon polyvinylidene difluoride membrane, excised, and hydrolyzed in acid. The phosphoamino acids thus liberated were separated by electrophoresis in the first dimension (1D) and ascending chromatography in the second dimension (2D). After migration, radioactive molecules were detected by autoradiography (lower panel). Authentic phosphoserine (P-Ser), phosphothreonine (P-Thr), and phosphotyrosine (P-Tyr) were run in parallel as internal standard controls, and visualized by ninhydrin staining (upper panel).