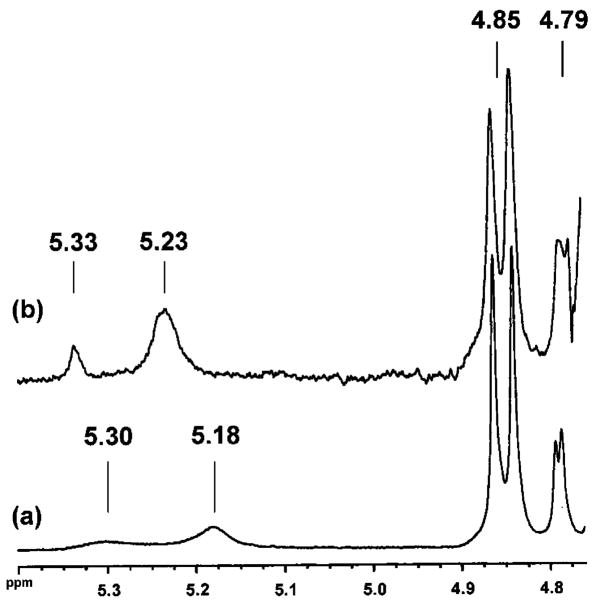
Figure 5.
Expansion of the 1H NMR spectra of solutions of (a) D-ribose and (b) a mixture of 1 and D-ribose (0.15 M NaOD/D2O). The resonances shown correspond the anomeric protons of each of the four cyclic forms. The assignments are δ (ppm): 5.30 (α-ribofuranose), 5.18 (β-ribofuranose), 4.85 (β-ribopyranose), and 4.79 (α-ribopyranose). Upon the addition of 1 only the resonances corresponding to the ribofuranoses exhibit a downfield shift. This is expected as a result of cyclic boronate formation with ribofuranose (ref 3c).