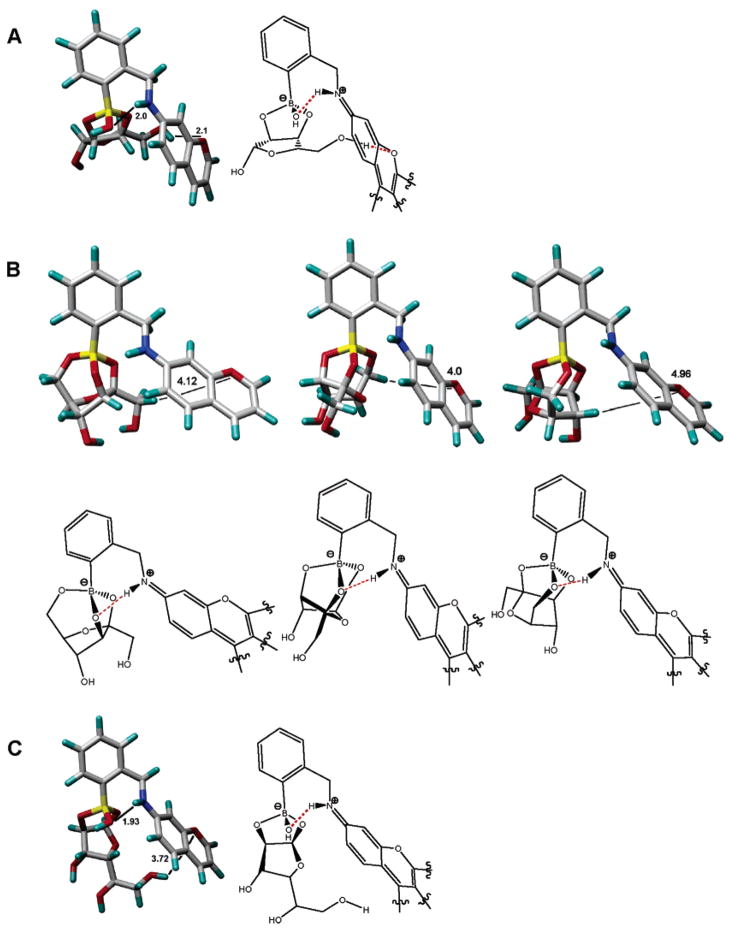
Figure 9.
Energy-minimized structures of boronates derived from 1 and ribofuranose (“endo” isomer, structure A), fructofuranose (three rotamers, structures B), and glucofuranose (“exo” isomer, structure C; the complementary conformers of the ribo- and glucofuranose boronates are included in the Supporting Information). A subunit of the rhodamine chromophore moiety is shown for clarity and used in the simulations in order to simplify the calculations. The above-calculated structures show that the ribofuranose complex exhibits the relatively best geometry for promoting direct contacts between the bound sugar moiety and the chromophore moiety of 1. Studies aimed at evaluating the specific interactions between ribose, adenosine, ribosides, and ribotides with 1 that might involve π–π stacking, σ–π interactions, and/or charged hydrogen bonding between the sugar and the rhodamine carboxylate functionality are ongoing.