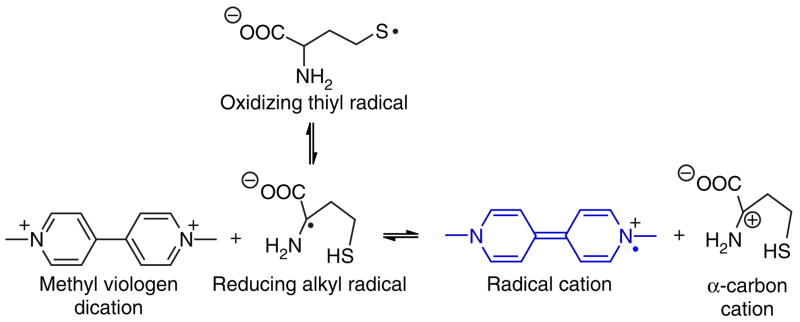
Figure 1.
Hcy undergoes a thiyl radical to carbon radical rearrangement more readily than other biothiols. This is attributed to the favored formation of a five-membered ring (H-atom transfer) transition state, as opposed to four-membered and nine-membered ring transition states as in the cases of cysteine and glutathione, respectively. Captodative stabilization of the alpha-amino carbon-centered radical renders the alpha C–H bond weaker than the S–H bond by ~ 4 kcal/mol. Alpha-amino-acid carbon radicals are well-known strong reductants. Favored formation of the reducing alpha-amino radical in the case of Hcy allows for the selective reduction of weakly oxidizing chromogens, such as methyl viologen dication at neutral pH.