Table 1. Heterocycle Synthesis by Functionalization of the Linker, Metathesis, and Release (See Scheme 1 for the Definitions of RF and R′F).
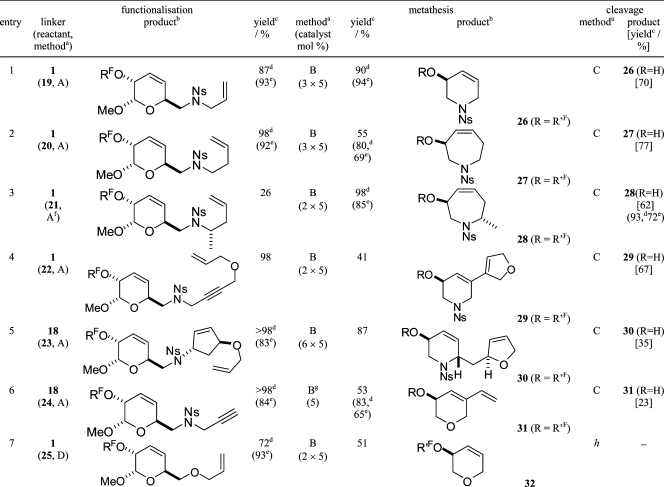 |
Method A: reactant (4 equiv), PPh3 (4 equiv), DEAD (4 equiv), THF, rt then F-SPE. Method B: (i) Hoveyda−Grubbs second generation catalyst, CH2Cl2, reflux; (ii) P(CH2OH)3, Et3N then silica; (iii) F-SPE. Method C: 3% TFA in CH2Cl2, rt then F-SPE. Method D: (i) NaH, THF, 0 °C; (ii) allyl bromide, rt; (iii) MeOH then F-SPE;
See Scheme 1 for the definitions of RF and R′F.
Unless otherwise stated, isolated yield of product.
Mass of product after F-SPE.
Purity (%) determined by HPLC after F-SPE.
10 equiv of the sulfonamide, PPh3, and DEAD were used.
In the presence of an ethylene atmosphere.
Not undertaken.
