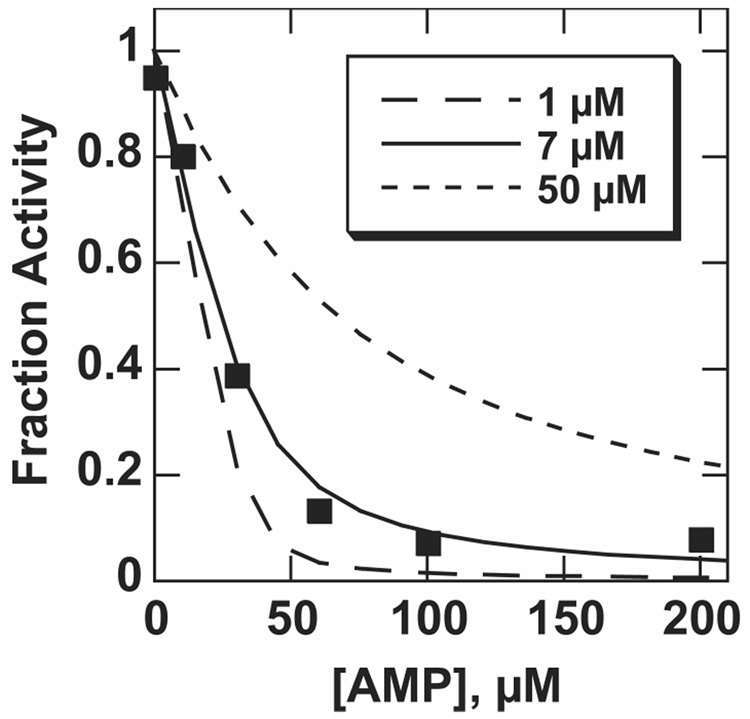
Figure 2.
Inhibition of pNPS hydrolysis with AMP at pH 6.5. Because high enzyme concentrations were used, the standard expression for competitive inhibition could not be used for pNPS inhibition (see Materials and Methods). Curves shown were calculated using the indicated value of Ki and the expression described in Materials and Methods. Data for this experiment were collected with [E] = 34 µM, [S] = 9.8 mM, in 0.1 M MES, pH 6.5, 0.5 M NaCl, 100 µM ZnCl2, at 25 °C. The Ki measured for MepNPP at pH 6.5 and in the same buffer conditions was 6.9 ± 0.2 µM (data not shown), and the pNPS inhibition data fit the inhibition expression with a Ki value of 6.1 ± 1.4 µM.