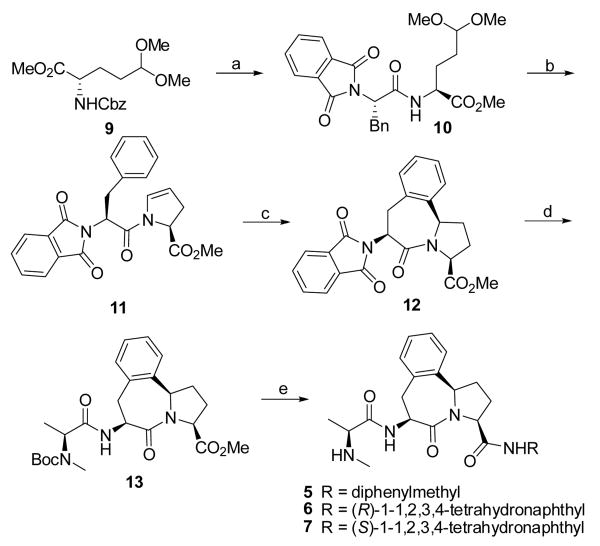
Scheme I.
Synthesis of compounds.
5-7. Reagents and conditions: (a) i. H2, 10% Pd-C, methanol; ii. N-phthaloyl-L-phenylpropanoic acid, EDC, HOBt, N-methylmorpholine, CH2Cl2-DMF 1:1, 0°C - rt, overnight, 92% over two steps; (b) CF3COOH, 4 Å molecular sieve, CHCl3, reflux, 88%; (c) trifluorosulfonic acid, trifluorosulfonic anhydride, CH2Cl2, 98%; (d) i. hydrazine hydrate, methanol, 3 days; ii. L-N-Boc-N-methyl-alanine, EDC, HOBt, N-methylmorpholine, CH2Cl2-DMF 1:1, 0 °C - rt, overnight, 92% over two steps; (e) i. 2 N LiOH, then 1 N HCl; ii. amine, EDC, HOBt, N-methylmorpholine, CH2Cl2-DMF 1:1, 0 °C - rt, overnight; iii. ZnBr2, CH2Cl2.