Table 1.
Preparation of the Requisite Terminal Alkynes a
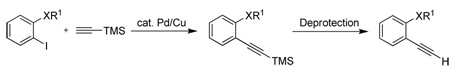 | |||||
|---|---|---|---|---|---|
| entry | XR1 | Coupling conditionsb |
TMS-alkyne (% yield) |
Deprotection conditionsc |
Terminal alkyne (% overall yield) |
| 1 | SMe | A | 1 (97) | aq KOH/MeOH 0.5 h/25 °C | 5 (83) |
| 2 | CO2Me | A | 2 (100) | KF-2H2O/MeOH 36 h/25 °C | 6 (82) |
| 3 | Ph | A | -d | - | - |
| 4 | CONHPh | B | 3 (80) | KF-2H2O/MeOH 0.5 h/25 °C | 7 (71) |
| 5 | NMe2 | C | 4 (74) | Aq NaOH/MeOH/ether 0.16 h/25 °C | 8 (48) |
All reactions have been run with 5 mmol of o-iodoarene.
Reaction conditions: (A) 1.2 equiv of alkyne, 2 mol % PdCl2(PPh3)2, 1 mol % CuI, 20 mL of Et3N, 25 °C. (B) 1.3 equiv of alkyne, 3 mol % PdCl2(PPh3)2, 2 mol % CuI, 4 equiv of DIPA, DMF, 65 °C. (C) 1.2 equiv. of alkyne, 1 mol % PdCl2(PPh3)2, 3 mol % CuI, 0.9 equiv of Et3N, DMF, 25 °C.
See the Supporting Information for the experimental details.
A complex reaction mixture was obtained.
