Table 2.
Preparation of the Diarylalkynes a
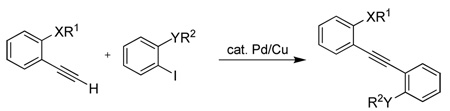 | ||||||
|---|---|---|---|---|---|---|
| entry | XR1 | YR2 | time (h) | temp (°C) | product | yield (%) |
| 1 | SMe | SeMe | 24 | 25 | 9 | 80 |
| 2 | SMe | OMe | 6 | 60 | 10 | 90 |
| 3 | SMe | CO2Me | 14 | 25 | 11 | 98 |
| 4 | SMe | CONHPh | 1 | 25 | - | -b |
| 5 | SMe | NMe2 | 24 | 25 | - | -c |
| 6 | CO2Me | SeMe | 10 | 60 | 12 | 87 |
| 7 | CO2Me | CONH2 | 2 | 25 | 13 | 83 |
| 8 | CO2Me | CONHPh | 4 | 60 | 14 | 62d |
| 9 | CO2Me | NMe2 | 8 | 25 | 15 | 78 |
| 10 | CO2Me | OMe | 4 | 25 | 16 | 74 |
| 11 | CO2Me | CHO | 14 | 25 | 17 | 84 |
| 12 | CO2Me | COMe | 4 | 25 | 18 | 88 |
| 13 | CO2Me | Ph | 2 | 25 | 19 | 70 |
| 14 | CONHPh | SMe | 3 | 65 | - | -b,e |
| 15 | CONHPh | SeMe | 18 | 65 | - | -c,e |
| 16 | NMe2 | Ph | 3 | 75 | 20 | 35 |
| 17 | NMe2 | Ph | 2 | 110 | 20 | 74f |
| 18 | OMe | NMe2 | 24 | 25 | 21 | 70 |
| 19 | OMe | CHO | 7 | 25 | 22 | 80 |
| 20 | OMe | OBn | 8 | 60 | 23 | 72 |
| 21 | OMe | Ph | 2 | 25 | 24 | 94 |
| 22 | CHO | NMe2 | 6 | 60 | - | -b |
All reactions were run using 1 mmol of iodoarene and 1.2 equiv of alkyne, 2 mol % PdCl2(PPh3)2, 1 mol % CuI, and 5 mL of Et3N.
An inseparable mixture was obtained.
The desired compound was not observed.
Four mol % of CuI was used.
Two milliliters of DMF were used to dissolve the alkyne.
The reaction was carried out in toluene using a modified procedure; see the Supporting Information.
