Table 3.
Results of the competitive cyclizations a
| Entry | Substrate | Electrophile | Time (h) | product(s) | Yield (%) |
|---|---|---|---|---|---|
| 1 | 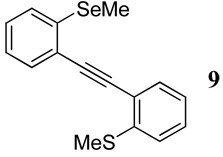 |
1.1 I2 | 0.16 | 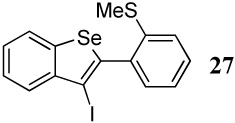 |
82b |
| 2 | 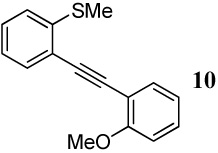 |
1.2 I2 | 1 | 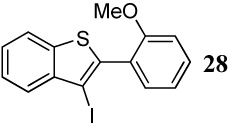 |
96 |
| 3 | 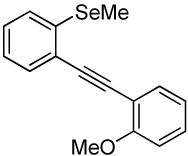 |
1.1 I2 | 0.5 | 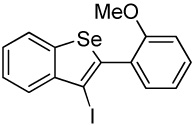 |
91c |
| 4 | 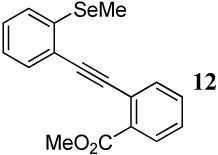 |
1.2 I2 | 1 | 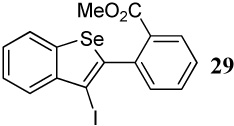 |
98d |
| 5 | 12 | 1.2 ICl | 29 | 93d | |
| 6 | 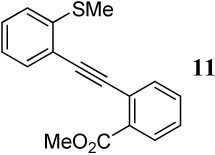 |
1.2 I2 | 1 | 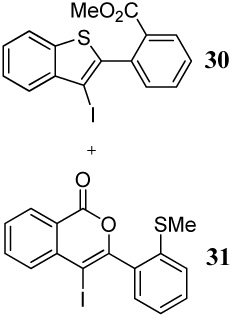 |
93 |
| 7 | |||||
| 7 | 11 | 1.2 ICl | 0.5 |
30 + |
93 |
| 31 | 7 | ||||
| 8 | 11 | 1.5 PhSeCl | 1 | 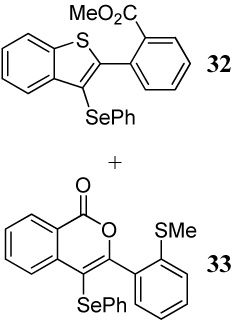 |
56e |
| 44e | |||||
| 9 |  |
1.2 I2 | 1 | 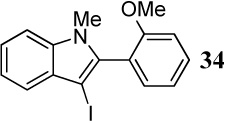 |
95 |
| 10 | 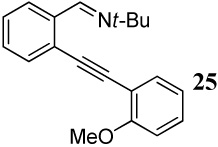 |
2 PhSeCl | 3 | 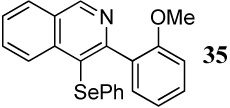 |
70 |
| 11 | 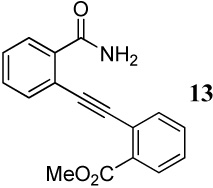 |
1.2 I2 | 1 | - | -f |
| 12 | 13 | 3 I2 | 1 | - | -f,g |
| 13 | 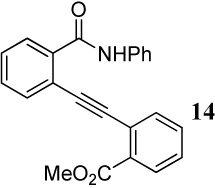 |
1.2 I2 | 1 | 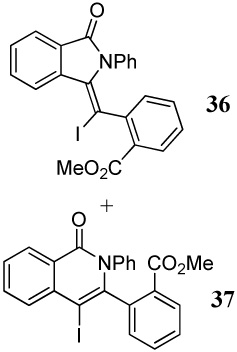 |
45 |
| 18 | |||||
| 14 | 14 | 1.2 ICl | 1 |
36+ |
35 |
| 37 | 30 | ||||
| 15 | 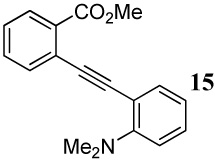 |
1.2 I2 | 1.5 | 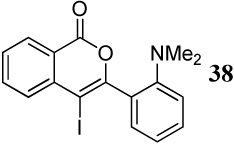 |
57h |
| 16 | 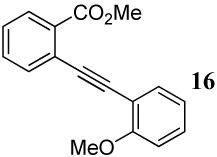 |
1.2 I2 | 1 | 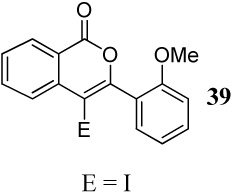 |
98 |
| 17 | 16 | 2 ICl | 0.5 | 39 | 96 |
| 18 | 16 | 1.5 PhSeCl | 0.5 | E = SePh 40 |
95 |
| 19 | 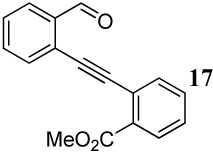 |
1.2 I2 | 2 | - | -i |
| 20 | 17 | 1.2 ICl | 0.5 | 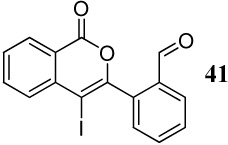 |
25j |
| 21 | 17 | 1.2 I2, 1.2 MeOH/1.0 K2CO3 |
10 | 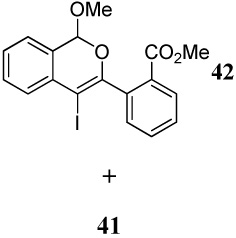 |
82k |
| 12 | |||||
| 22 | 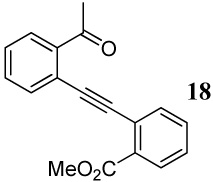 |
1.2 I2 | 1 | - | -f |
| 23 | 18 | 1.2 ICl | 0.5 | - | -f |
| 24 | 18 | 1.2 I2, 1.2 MeOH/1.0 K2CO3 |
2 | - | -f |
| 25 | 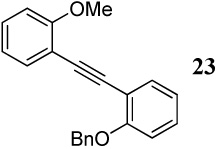 |
2 I2 | 3 | 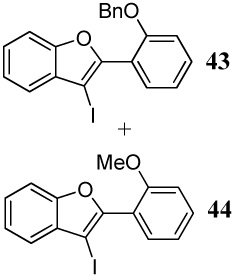 |
4:1l |
| 26 | 23 | 1.2 ICl | 3 | 43 + 44 | 6:1l |
| 27 | 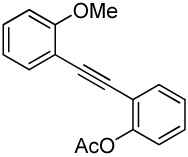 |
2 I2 | 3 | 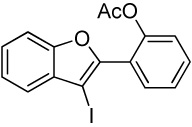 |
95m |
| 28 | 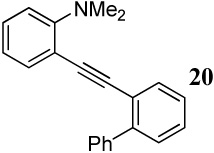 |
2 I2 | 2 | 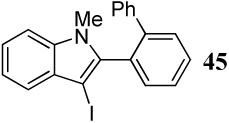 |
78 |
| 29 | 20 | 1.2 ICl | 0.5 | - | -f |
| 30 | 20 | 1.2 NBS | 1 | - | -f |
| 31 | 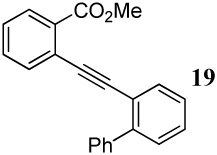 |
2 ICl | 3 | 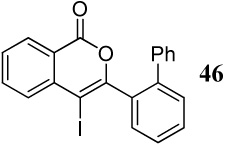 |
90 |
| 32 | 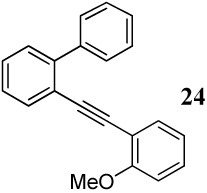 |
2 I2 | 3 | - | -f |
| 33 | 24 | 1.2 ICl | 0.5 | 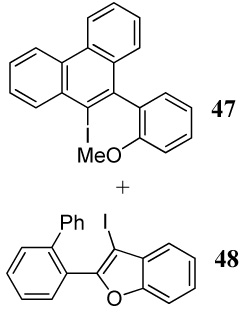 |
49e |
| 33e | |||||
| 34 | 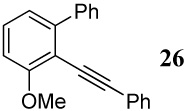 |
6 I2 | 2 | 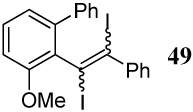 |
89d |
| 35 | 26 | 1.2 ICl | 1 | - | -d,n |
Unless otherwise stated, all reactions have been carried out on a 0.25 mmol scale in 5 ml of methylene chloride at room temperature. All yields are isolated yields after column chromatography.
A small amount of the corresponding benzothiophene product (~7%) was observed by GC-MS analysis; however, it could not be isolated.
This result has previously been reported in the literature (see reference 8a).
This reaction hasf been carried out on a 0.10 mmol scale.
The reported yield is the average of two runs.
This reaction resulted in a complex mixture of unidentifiable products.
MeCN was used as the reaction solvent and 3 equiv of NaHCO3 were added as a base.
The corresponding indole (~8%) was also observed by GC-MS analysis. However, it could not be isolated.
No reaction occurred; the starting material was recovered. A complex mixture was obtained upon longer reaction.
This was the only isolated product. The rest of the product mixture was complex and inseparable.
This product decomposed quickly; see the Supporting Information for details.
An inseparable complex mixture was obtained. This ratio is based on GC-MS data.
This result has previously been reported in the literature (see reference 3b).
An alkyne ICl addition product whose structure is similar to compound 49 in entry 34 was obtained.
