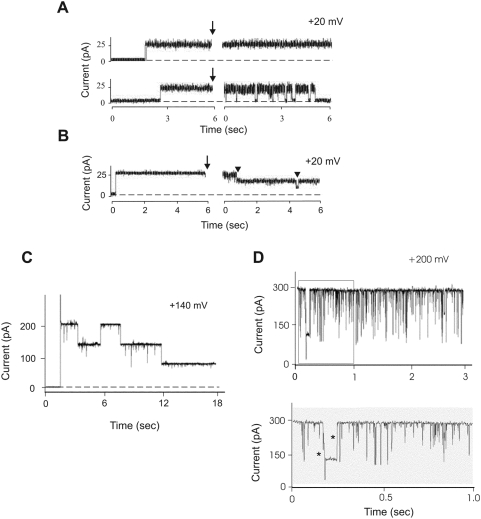
Figure 4. Sub-conductance states of the Pxmp2 channel.
(A) Effect of anti-Pxmp2 antibodies on the pore-forming activity of Pxmp2. The results presented on panels A–D were collected on Pxmp2 channels at fully open state; the data were filtered at 0.4 kHz (A and B) or at 1.0 kHz (C and D) and recorded at 2.0 kHz. Single Pxmp2 channel was incorporated into lipid bilayer and analyzed before and after addition (marked by arrow) of the IgG fraction (3 µg) isolated from pre-immune (upper trace) or immune (lower trace) serum to both compartments of the chamber. The time gap is 120 sec. Here, and on panel B, experiments were repeated 6 times, typical pictures are presented. (B) Effect of alkalization of the bath solution on the pore-forming activity of Pxmp2. Current traces of a bilayer containing one Pxmp2 pore-forming protein before and after addition (marked by arrow) of 0.2 M Na2CO3, pH 11.2 (150 µl) to both compartments of the chamber are shown. The time gap is 60 sec. Two steps of the channel closure are marked by arrowheads. (C) High holding potential leads to closure of the Pxmp2 channel. The artificial membrane contained a single high-conductance channel (the insertion event not shown). Note stepwise closure of the channel at high holding potential, the amplitude of each step constitutes one third of that one of the fully open channel. (D) Flickering of a single Pxmp2 channel incorporated into lipid bilayer. The lower trace shows a timescale-expanded recording from the part (in the frame) of the upper trace. The direct transitions between three main subconductance states are marked by asterisks.